Abstract
This work reports a part of hydroponic experiment results concerning changes in Salix viminalis L. cv. ‘Cannabina’ morphology and physiology under stress conditions with different copper concentration levels and verifies our earlier results about the role of different Ca/Mg ratios in trace elements’ accumulation efficiency. In this part, we present the copper accumulation and changes in willow biomass. Concentration of copper in roots, rods, shoots and leaves was analyzed with flame atomic absorption spectrometry. Selected indices characterizing copper accumulation and plant biomass structure were calculated to estimate the potential of willow to remove metal from polluted solution. Our results indicate a general increase of copper accumulation by selected willow organs with increase of copper concentration in modified Knop’s medium. Moreover, significant differences in copper phytoextraction between plants under different Ca/Mg ratios were affirmed (1:10 > 4:1 > 20:1 > 1:1/4).
Similar content being viewed by others
Introduction
Copper is an essential element necessary in the regular function of living organisms, including plants. Copper is taken up by roots and leaves in cation Cu2+ form or complex form with organic ligands (Sahi et al. 2007). In green plant organs, copper is present in chloroplasts and it is a component of many enzymes which catalyze oxidation reactions with the use of O2 (Borghi et al. 2008; Punniyamurthy and Rout 2008). Dynamics of copper phytoextraction are usually lower than for other trace elements. The optimum copper concentration is between 3 and 20 mg kg−1 and concentrations above 30 mg kg−1 are toxic for the majority of plants (Kabata-Pendias and Pendias 1999).
The significant amount of copper in the environment is the result of different methods of removing this metal or obtaining it in pure form (phytomining) (Robinson et al. 1997; Brooks et al. 1998). For many years, an increase in the number of studies focused on trace elements’ phytoextraction efficiency has been observed (Van Nevel et al. 2007; Gabos et al. 2009; Liang et al. 2009). Increase of phytoextraction efficiency is achieved by the use of new plants (including genetically modified plants) (Kotrba et al. 2009; Kuzovkina and Volk 2009; Kavamura and Esposito 2010; Kärenlampi et al. 2000), addition of selected chemical compounds complexed with trace element ions (Wang et al. 2009; Kim and Lee 2010), modification of growth conditions (Leštan et al. 2008; Safari Sinegani and Khalilikhah 2011) and use of plants with significant biomass production (Weatherall et al. 2006; Hernández-Allica et al. 2008; Abhilash and Yunus 2011).
A significant factor determining plant growth as well as trace elements’ phytoextraction efficiency is occurrence (Nair et al. 2008) and concentration levels of macro- and microelements in soil (interaction) (Fargašová and Beinrohr 1998; Walker and Bernal 2003; Ke et al. 2007). Excess of one element with respect to another element usually is the cause of the physiological response of the plant (Benzarti et al. 2008; Guala et al. 2011). The mutual macroelements ratio in soil has a significant role in plant growth, as presented by Haynes (1990) with regard to the significant inhibition of calcium uptake in case of higher concentrations of Mg2+, K+ or \( {\text{NH}}_{4}^{+} \). The elements calcium and magnesium fulfill a physiological role and their concentrations in soil, as well as ratio, are significant for plant growth (Weatherall et al. 2006; Mleczek et al. 2011a). Calcium is an essential nutrient component regulating uptake and translocation of other elements and compounds (Wójcik 1998; White and Broadley 2003). Moreover, presence of calcium amplifies plant resistance to pathological factors and gives the correct structure and cell hydration (Scrase-Field and Knight 2003). Magnesium is an essential component of chlorophyll and an activator of many enzymes. This element is taken up by plants as Mg2+cations and is more mobile than Ca2+, but less mobile than K+ (Kabata-Pendias and Pendias 1999).
The purpose of this work was to determine the copper accumulation efficiency and changes in willow biomass in relation to different Ca/Mg ratios and copper concentration levels.
Materials and methods
Experiment design
One-year-old cuttings of Salix viminalis L. cv. ‘Cannabina’ collected from three-year old rootstock without foliage were used in the experiment. To induce root formation, standardized rods (25 cm long, 18 mm in diameter) were incubated in modified Knop’s medium: (4.24 mM of Ca(NO3)2, 2.5 mM of KNO3, 1.84 mM of KH2PO4, 1 mM of MgSO4 7H2O, 0.045 mM of FeSO4 7H2O and microelements: 10 μM NaFeEDTA, 6.25 μM H3BO3, 0.5 μM MnCl2, 0.5 μM ZnSO4, 0.025 μM CuSO4, 0.125 μM Na2MoO4, 1.25 μM KJ, 0.025 μM CoCl2 diluted to 1 L (Barabasz et al. 2010). Nutrient solution was adjusted to pH 5.8 (PN-ISO 10390:1997) and electrolytic conduction (PN-ISO 1265 + AC1: 1997) of nutrient solution was 1.63 ms cm−1.
Concentrations of the most significant ions were as follows: 4.23 mM of Ca2+, 1.04 mM of Mg2+, 6.63 mM of NO3 −, 1.84 mM of PO4 3−, 4.32 mM of K+, and 1.06 mM of SO4 2−. To improve root formation, the solution applied at the beginning of the preliminary incubation period contained 50 % of salt contents in the standard Knop medium, which also facilitated easier and faster adaptation of rods to the new conditions. After 10 days, plants were selected according to the similar size of the root system (length and amount of roots) to obtain a uniform group and transferred into the Knop’s medium (0.5 L) containing copper Cu(NO3)2 × 3H2O salt at 0 (Cu0), 1.0 (Cu1), 2.0 (Cu2) and 3.0 (Cu3) mM addition levels (Table 1).
Willow rods were cultivated in hydroponic pots (13 × 15, diameter × height), stabilized by ultra-pure river sand (1.24 kg per pot: pH = 7.13, SiO2 content > 97 %, moisture content 0.058 %) with 3 different Ca/Mg ratios:
1:1/4 where CCa = 1.04 mM and CMg = 0.26 mM,
20:1 where CCa = 21.16 mM and CMg = 1.06 mM,
1:10 where CCa = 1.06 mM and CMg = 10.6 mM,
and as the reference (control) Ca/Mg 4:1 ratio (CCa = 4.23 mM and CMg = 1.06 mM). Ca and Mg ions were added to modified Knop solution in the form of two soils: Ca(NO3)2 and MgSO4. The use of particular Ca/Mg ratios was not accidental. We were interested in plant response when Ca/Mg ratio is 4:1 (physiological ratio), 1:1/4 (deficiency of two macroelements), 20:1 and 1:10 (calcium or magnesium in excess). Plants were cultivated in hydroponic pots with one willow cutting per pot and seven plants per each Cu concentration and each Ca/Mg ratio. Humidity of ultra-pure river sand in hydroponic pots was controlled by plastic float which allowed addition of proper water amount .
The 21-day experiment was conducted in a climate chamber under controlled conditions (21-15 ± 1 °C day/night temperature, relative humidity 79 ± 1 %), equipped with a fluorescent lamp (TL-D 36 W/865 G13 Philips) providing a radiation (photon) flux of 255 μE sec−1 m−2 (μmol sec−1 m−2) at the top of the plant for 16 h a day.
Plant materials preparation
Roots, rods, bark (separated mechanically and isolated from rods), shoots and leaves of willow plants were carefully washed with distilled water, dried in an electric drier at 105 ± 5 °C for 72 h, and then dry samples were ground to a powder for 3 min in a laboratory Cutting Boll Mill PM 200 by RETSCH. The material as three representative samples (3 g each) was mineralized in a CEM Mars 5 Xpress microwave mineralization system (CEM, Matthews, NC, USA) in a closed system (55 mL vessels) using 8 mL concentrated HNO3 and 2 mL 30 % H2O2. Digestion of the plant materials was performed according to a microwave program composed of three stages: first stage—power 600 W, time 5 min, temperature 120 °C; second stage—power 1200 W, time 10 min, temperature 160 °C; third stage—power 1600 W, time 10 min, temperature 200 °C. Materials after digestion were filtered through 45-mm filters (Qualitative Filter Papers Whatman, Grade 595: 4–7 μm), and then whole contents were made up to a final volume of 50 mL with deionized water (Milli-Q Academic System (non-TOC)).
Cu concentration in the willow organs leaves, bark, shoots, rods and roots was analyzed with flame atomic absorption spectrometry (FAAS) using a Agilent Technologies AA Duo - AA280FS/AA280Z spectrometer (Agilent Technologies, Mulgrave, Victoria, Australia) equipped with a Varian hollow-cathode lamp (HCl). Calibration curves were prepared before the analysis with six replicates per each Cu concentration out of stock solution of 1 g dm−3 (FLUKA). Results were validated on the basis of certified reference materials, i.e. NIST 1575a (pine needles) from National Institute of Standards and Technology, Gaithersburg, NCS DC 73350 (leaves of poplar) and NCS DC 73,349 (bush branches and leaves) both from China National Analysis Center for Iron and Steel, Beijing, China, analyzed in every tenth determination set (Table 2).
Simultaneously, analysis of randomly selected samples using inductively coupled plasma-optical emission spectrometry (ICP-OES) with Vista MPX apparatus by Varian was performed.
Efficiency of copper phytoextraction was characterized by BAF values calculated as the ratio of trace element concentration in willow organs to the concentration of this metal in solution (Eq. 1).
Depending on BAF values, accumulation efficiency was estimated using one of four groups: BAF > 1 (I—intensive), 1–0.1 (M—medium), 0.1–0.01 (W—weak) and 0.01–0.001 (L—no accumulation) (Kabata-Pendias and Pendias 1999).
According to Maiti and Jaiswal (2008), the efficiency of copper ion transport from roots to harvestable aerial plant organs was estimated by the translocation factor (TF) and transfer factor (TFr) values. These factors were calculated according to the following equations (Eqs. 2, 3):
TF and TFr values were calculated to complete information about copper ion transport from medium to plant (TF) and to determine metal translocation in plant organs (TFr).
To better characterize the copper accumulation rate in the whole experiment, the total metal accumulation rate (TAR) values were calculated according to formula (Eq. 4) presented in numerous studies such as: Ait Ali et al. (2004), Mohanty and Patra (2011) and also Aksorn and Chitsomboon (2013).
Biometric analysis
At the beginning and at the end of the experiment, the length of shoots, leaves and roots and also total leaf surface area were measured. In addition, root biomass and the whole plant biomass were determined. The length of willow organs was analyzed directly by electronic tape Proline 20387. Leaf area was estimated with a DOCUPEN RC 800 manual portable scanner with ABBYY FineReader 6.0 Sprint and Adobe Photoshop 10 software. Based on measurements of all leaves analyzed after and before the experiment, the increase of leaves area within experiment was presented.
To estimate the resistance of the tested willow taxon in copper phytoextraction, the tolerance index biomass (TIb) values for leaves and roots were calculated according to Eq. 5.
According to Wilkins (1978), we can calculate 3 values for TIb: TIb < 1 (a net decrease in biomass and a stressed condition of plants), TIb = 1 (no difference relative to control treatments) and TIb > 1 (a net increase in biomass and correct plant development). Due to the highest copper accumulation in willow roots, the tolerance index (TIr) values were also calculated according to Eq. 6:
Statistical analysis
The data were processed using Microsoft Excel 2010. Statistical analysis was done using STATISTICA 6 with two-way MANOVA and post hoc Tukey test at P = 0.05. Two-factor analyses of variance were used to examine the differences between the levels of copper concentration (0, 1, 2 and 3 mM) and between Ca/Mg ratios (20:1, 4:1, 1:1/4, 1:10) with regard to parameters (indices) characterizing accumulation efficiency and biomass of willow.
Results
Efficiency of copper phytoextraction by willow organs
In general, the exact increase of copper uptake by willow cuttings with increase of copper concentration in medium was affirmed (Fig. 1).
-
Efficiency of copper phytoextraction was Ca/Mg ratio dependent and for all willow organs was in the following order: 1:10 > 4:1 > 20:1 > 1:1/4,
-
In general, for the copper concentration in the medium and Ca/Mg ratio, efficiency of this metal uptake by willow organs was as follows: roots > rods > shoots ≥ bark > leaves.
To estimate copper phytoextraction efficiency dependent on copper concentration in medium, the BAF values were calculated and results are presented in Table 3.
In the majority of cases, the copper accumulation in willow organs was estimated as weak (BAF = 0.1–0.01). Medium accumulation depending on copper concentration in all willow organs except leaves in 1:10 Ca/Mg ratio was observed. Medium copper accumulation was also affirmed in willow roots under the lower metal concentrations in medium and at different Ca/Mg ratios.
To determine copper ion transport from medium to plant and this metal’s translocation inside the plant, the TFr and TF were calculated. In addition, to assess phytoextraction dynamics in the studied willow taxon, the total accumulation rate index (TAR) was calculated. The characteristics of these indices are presented in Table 4.
Results presented in Table 4 indicate restricted copper ion transport from Knop medium to plant. Along with copper concentration increase in solution, transfer factor values were decreased. Based on the results of copper accumulation efficiency by particular willow organs, it is possible to state that despite sorption increase with copper concentration in Knop solution, the amount of accumulated metal in relation to available metal decreased.
TF values pointed to significant diversity in relation to Ca/Mg ratio and copper concentration in Knop solution. The highest values were for plants grown on under 2 mM (Cu2) copper mixture, lower under 3 mM (Cu3), with the exception of 1:10 Ca/Mg ratio, and lowest for plants treated by 1 mM (Cu1). These values indicate that the translocation of copper ions was mainly dependent on concentration of this metal.
TAR values pointed to the similar relationships as for TF and simultaneously the diverse speed of copper sorption, more dependent on copper accumulation than Ca/Mg ratio (statistically significant differences were observed especially between three Ca/Mg ratios, 20:1, 4:1 and 1:10, for all copper concentrations in medium).
Biometric analysis
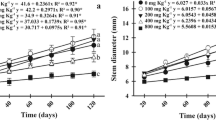
Characteristics of morphological changes in tested willow rods are presented in Fig. 2.
For particular analyzed parameters, significant differences were observed only between selected Ca/Mg ratios and Cu concentrations. In the case of shoot length increase, a similar tendency of cuttings growth was observed for plants under 20:1, 1:1/4 and 1:10 Ca/Mg ratios and it was other than under 4:1 treatment Ca/Mg ratio. A similar tendency (decrease of selected parameter with Cu concentration increase) was observed especially for 20:1 Ca/Mg ratio for total leaf surface area, root biomass and total plant biomass. The results of changes in leaf length indicated that even greater changes were mainly Ca/Mg ratio dependent. In the case of root biomass, the changes were similar to the ones observed for total plant biomass and this parameter was characterized by the tolerance index.
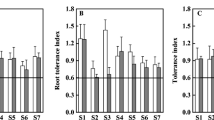
Tolerance index (TI) was calculated to estimate plant biomass in relation to different copper concentration levels and Ca/Mg ratio (Table 5).
Tolerance index values calculated for total plant biomass (TIb) were insignificantly diverse in relation to copper concentration in medium or Ca/Mg ratio. TIb values for three Ca/Mg ratios (4:1, 1:1/4 and 1:10) and two copper concentrations in mixture (Cu1 and Cu2) were above 1. This indicates stimulation of plant biomass growth. In the rest of the cases, especially plants under Cu3 in each Ca/Mg ratio, all TIb values were below 1, which indicates limitation of plant biomass production. In addition, due to roots showing the highest copper sorption efficiency in the experiment, the tolerance index values for willow root length (TIr) were estimated. In this case, TIr values higher than 1 were observed in plants under all three copper concentration levels (Cu1, Cu2, Cu3) and two Ca/Mg ratios (4:1 and 1:1/4). In the rest of the cases, the root length was limited.
Discussion
The results focused on efficiency of copper phytoextraction and biomass growth by willow rods. Our observations are similar to the other studies and are a basic source for further consideration indicating physiological changes and possibilities to use tested willow taxa in phytoremediation. Borghi et al. (2007) tested phytoextraction efficiency with use of poplar woody cuttings (Populus × euramericana) clone Adda under experimental copper concentration levels (0.0004 (control), 0.02, 0.1, 0.5 and 1 mM) in a hydroponic experiment (Hoagland’s solution). The results of our experiment confirm the general reduction of plant growth under copper concentrations higher than 0.1 mM. A similarity was also observed of copper translocation in plant (accumulation mainly in roots). The authors in this work indicate that the poplar clone Adda is able to tolerate quite high copper concentrations. We can state a similar conclusion, because growth of willow rods tested in our work was decreased but simultaneously copper accumulation efficiency, despite a decrease of TAR values, was significantly increased. We have also tested other willow taxa and we used a higher copper concentration (unpublished data). It suggests that the results of changes in plant biomass under similar ranges of copper concentration presented by many authors are similar in spite of different plants used (Mal et al. 2002).
Somewhat different observations were presented by Bouazizi et al. (2010), who studied copper toxicity in expansion of Phaseolus vulgaris L. leaves. The presented results pointed to copper sorption increase in plants grown in control solution and with 50 μM Cu in nutrient solution. Efficiency of copper sorption in plants under 75 μM was lower than 50 μM and control. The dry weight production was lower, the higher was the copper concentration in the nutrient solution. These results also confirmed our results and indicate at the same time the narrow limit where increase or decrease of accumulation/biomass production is observed.
When comparing our results and results presented by Dos Santos Utmazian et al. (2007), willow growth and phytoextraction efficiency were significantly plant species dependent. We can suggest that some other willow species/varieties that we tested in our other work (Mleczek et al. 2010) may be most interesting to use in phytoextraction of copper polluted areas. In addition, Dos Santos Utmazian et al. (2007) reported differences in plant biomass, metal tolerance and metal phytoextraction between willow clones. This fact explains the way of copper translocation and differences in results presented in some studies cited or not cited in this work. The tolerance index (TI) values for total plant biomass (TIb) and roots (TIr) presented in our work were significantly lower than those presented by Dos Santos Utmazian et al. (2007); however, they present plant growth inhibition or stimulation dependent on metal concentration in nutrient solution.
In our experiment, only for 20:1 Ca/Mg ratio and for 1:10 and 1:1/4 Ca/Mg ratios, a general decrease of shoot length was observed but with some exceptions. The results for physiological 4:1 Ca/Mg ratio did not confirm the results presented by Juang et al. (2011). This observation is a sign of the significant role of Ca/Mg ratio for willow growth. No significant differences in shoot growth between particular copper doses and significant differences between them and a medium without copper addition (0) confirm the high immunity of the tested willow taxon. In addition, the studies described by Juang et al. (2011) indicate significant inhibition of the plant growth rate with metal concentration increase in the nutrient solution. The same results were also observed in our experiment but with use of a higher copper concentration in Knop solution.
The results of the influence of different Ca/Mg ratios confirmed the results presented by us in our earlier experiments (Magdziak et al. 2011; Mleczek et al. 2011b). We have not found the same form of experiment, so a wider discussion in this area is difficult.
Conclusion
Studies of plants with high trace element phytoextraction efficiency are among the most frequent subjects of environmental works. A lot of them are focused only on the highest efficiency of this process or the highest plant biomass. That is the correct approach but we cannot forget about the plant and its response to stress aroused by the presence of trace elements. In this work, we have presented only two aspects of willow use (phytoextraction and biomass growth). The results indicate medium potential of this plant for use in phytoremediation (increase of phytoextraction but decrease of biomass growth). Nonetheless, the willow taxon presented in our work has a small habitat requirement and it readily adapts to new environmental conditions. Moreover, within 21 days of the experiment we did not observe any chlorosis or necrotic changes. We know that the results from hydroponic experiments are somewhat artificial and sometimes are significantly different than results from field experiments. Nevertheless, we have tested this and over 150 other willow taxa in both field and hydroponic experiments, which has given us fundamental knowledge about use of willow in natural ecosystems.
Authors contribution
Mirosław Mleczek contributed to copper analysis, experiment preparation and biometric analysis. Monika Gąsecka contributed to experiment preparation and plant material preparation. Kinga Drzewiecka contributed to statistical analysis. Piotr Goliński contributed to manuscript preparation. Zuzanna Magdziak contributed to experiment preparation. Tamara Chadzinikolau contributed to manuscript preparation.
References
Abhilash PC, Yunus M (2011) Can we use biomass produced from phytoremediation? Biomass Bioenerg 35:1371–1372. doi:10.1016/j.biombioe.2010.12.013
Ait Ali N, Bernal MP, Ater M (2004) Tolerance and bioaccumulation of cadmium by Phragmites australis grown in the presence of elevated concentrations of cadmium, copper, and zinc. Aquat Bot 80:163–176. doi:10.1016/j.aquabot.2004.08.008
Aksorn E, Chitsomboon B (2013) The Effects of Plant Growth Promoting Traits on Heavy Metal Uptake of Vetiver Grasss. American-Eurasian J Agric Environ Sci 13:465–470
Barabasz A, Krämer U, Hanikenne M, Rudzka J, Antosiewicz DM (2010) Metal accumulation in tobacco expressing Arabidopsis halleri metal hyperaccumulation gene depends on external supply. J Exp Bot 61:3057–3067. doi:10.1093/jxb/erq129
Benzarti S, Mohri S, Ono Y (2008) Plant response to trace element toxicity: comparative study between the hyperaccumulator Thlaspi caerulescens (Ecotype Ganges) and nonaccumulator plants: lettuce, radish, and alfalfa. Environ Toxicol 23:607–616. doi:10.1002/tox.20405
Borghi M, Tognetti R, Monteforti G, Sebastiani L (2007) Responses of Populus × euramericana (P. deltoides × P. nigra) clone Addato increasing copper concentrations. Environ Exp Bot 61:66–73. doi:10.1016/j.envexpbot.2007.03.001
Borghi M, Tognetti R, Monteforti G, Sebastiani L (2008) Responses of two poplar species (Populus alba and Populus × canadensis) to high copper concentrations. Environ Exp Bot 62:290–299. doi:10.1016/j.envexpbot.2007.10.001
Bouazizi H, Jouili H, Geitmann A, El Ferjani E (2010) Copper toxicity in expanding leaves of Phaseolus vulgaris L.: antioxidant enzyme response and nutrient element uptake. Ecotoxicol Environ Saf 73:1304–1308. doi:10.1016/j.ecoenv.2010.05.014
Brooks RR, Chambers MF, Nicks LJ, Robinson BH (1998) Phytomining. Trends Plant Sci 3:359–362. doi:10.1016/S1360-1385(98)01283-7
Dos Santos Utmazian MN, Wieshammer G, Vega R, Wenzel WW (2007) Hydroponic screening for metal resistance and accumulation of cadmium and zinc in twenty clones of willows and poplars. Environ Pollut 148:155–165. doi:10.1016/j.envpol.2006.10.045
Fargašová A, Beinrohr E (1998) Metal-metal interactions in accumulation of V5+, Ni2+, Mo6+, Mn2+ and Cu2+ in under- and above-ground parts of Sinapis alba. Chemosphere 36:1305–1317. doi:10.1016/S0045-6535(97)00375-5
Gabos MB, de Abreu CA, Coscione AR (2009) EDTA assisted phytoremediation of Pb contaminated soil: metal leaching and uptake by Jack Beans. Scientia Agricola (Piracicaba, Brazil) 66:506-514. doi:10.1590/S0103-90162009000400012
Guala SD, Vega FA, Covelo EF (2011) Development of a model to select plants with optimum metal phytoextraction potential. Environ Sci Pollut Res Int 18:997–1003. doi:10.1007/s11356-011-0456-x
Haynes RJ (1990) Active ion uptake and maintenance of cation-anion balance: a critical examination of their role in regulating rhizosphere pH. Plant Soil 126:247–264. doi:10.1007/BF00012828
Hernández-Allica J, Becerril JM, Garbisu C (2008) Assessment of the phytoextraction potential of high biomass crop plants. Environ Pollut 152:32–40. doi:10.1016/j.envpol.2007.06.002
Juang KW, Lai HY, Chen BC (2011) Coupling bioaccumulation and phytotoxicity to predict copper removal by switchgrass grown hydroponically. Ecotoxicol 20:827–835
Kabata-Pendias A, Pendias H (1999) Biogeochemia pierwiastków śladowych. Biogeochemistry of trace elements, 3rd edn. Wyd. Naukowe PWN, Warsaw (in Polish)
Kärenlampi S, Schat H, Vangronsveld J, Verkleij JAC, van der Lelie D, Mergeay M, Tervahauta AI (2000) Genetic engineering in the improvement of plants for phytoremediation of metal polluted soil. Environ Pollut 107:225–231. doi:10.1016/S0269-7491(99)00141-4
Kavamura VN, Esposito E (2010) Biotechnological strategies applied to the decontamination of soils polluted with trace elements. Biotechnol Adv 28:61–69. doi:10.1016/j.biotechadv.2009.09.002
Ke W, Xiong ZT, Chen S, Chen J (2007) Effects of copper and mineral nutrition on growth, copper accumulation and mineral element uptake in two Rumex japonicas populations from a copper mine and an uncontaminated field sites. Environ Exp Bot 59:59–67. doi:10.1016/j.envexpbot.2005.10.007
Kim SH, Lee IS (2010) Comparison of the ability of organic acids and EDTA to enhance the phytoextraction of elements from a multi-metal contaminated soil. Bull Environ Contam Toxicol 84:255–259. doi:10.1007/s00128-009-9888-0
Kotrba P, Najmanova J, Macek T, Ruml T, Mackova M (2009) Genetically modified plants in phytoremediation of heavy metal and metalloid soil and sediment pollution. Biotechnol Adv 27:799–810. doi:10.1016/j.biotechadv.2009.06.003
Kuzovkina YA, Volk TA (2009) The characterization of willow (Salix L.) varieties for use in ecological engineering applications: co-ordination of structure, function and autecology. Ecol Eng 35:1178–1189. doi:10.1016/j.ecoleng.2009.03.010
Leštan D, Luo CL, Li XD (2008) The use of chelating agents in the remediation of metal-contaminated soils: a review. Environ Pollut 153:3–13. doi:10.1016/j.envpol.2007.11.015
Liang HM, Lin TH, Chiou JM, Yeh KC (2009) Model evaluation of the phytoextraction potential of trace element hyperaccumulators and non-hyperaccumulators. Environ Pollut 157:1945–1952. doi:10.1016/j.envpol.2008.11.052
Magdziak Z, Kozlowska M, Kaczmarek Z, Mleczek M, Chadzinikolau T, Golinski P, Drzewiecka K (2011) Influence of Ca/Mg ratio on phytoextraction properties of Salix viminalis. II. Secretion of low molecular weight organic acids to the rhizosphere. Ecotoxicol Environ Saf 74:33–40. doi:10.1016/j.ecoenv.2010.09.003
Maiti SK, Jaiswal S (2008) Bioaccumulation and translocation of elements in the natural vegetation growing on fly ash lagoons: a field study from Santaldih thermal power plant, West Bengal, India. Environ Monit Assess 136:355–370. doi:10.1007/s10661-007-9691-5
Mal TK, Adorjan P, Corbett AL (2002) Effect of copper on growth of an aquatic macrophyte, Elodea canadensis. Environ Pollut 120:307–311. doi:10.1016/S0269-7491(02)00146-X
Mleczek M, Rutkowski P, Rissmann I, Kaczmarek Z, Golinski P, Szentner K, Strażyńska K, Stachowiak A (2010) Biomass productivity and phytoremediation potential of Salix alba and Salix viminalis. Biomass Bioenerg 34:1410–1418. doi:10.1016/j.biombioe.2010.04.012
Mleczek M, Kozłowska M, Kaczmarek Z, Magdziak Z, Goliński P (2011a) Cadmium and lead uptake by Salix viminalis under modified Ca/Mg ratio. Ecotoxicol 20:158–165. doi:10.1007/s10646-010-0567-z
Mleczek M, Kozlowska M, Kaczmarek Z, Chadzinikolau T, Magdziak Z, Golinski P (2011b) Influence of Ca/Mg on phytoextraction properties of Salix viminalis. I. The effectiveness of Cd, Cu, Pb and Zn bioaccumulation and plant growth. Int J Phytorem 14:75–88. doi:10.1080/15226514.2011.573824
Mohanty M, Patra HK (2011) Attenuation of chromium toxicity in mine waste water using water hyacinth. J Stress Physiol Biochem 7(4):335–346. doi:10.1007/978-1-4419-7615-4_1
Nair A, Juwarkar AA, Devotta S (2008) Study of speciation of elements in an industrial sludge and evaluation of metal chelators for their removal. J Hazard Mater 152:545–553. doi:10.1016/j.jhazmat.2007.07.054
PN-ISO 10390:1997 Soil quality. Determination of pH
PN-ISO 1265 + AC1: 1997 Soil quality. Determination of electrolytic conduction
Punniyamurthy T, Rout L (2008) Recent advances in copper-catalyzed oxidation of organic compounds. Coord Chem Rev 252:134–154. doi:10.1016/j.ccr.2007.04.003
Robinson BH, Chiarucci A, Brooks RR, Petit D, Kirkman JH, Gregg PEH, De Dominics V (1997) The nickel hyperaccumulator plant Alyssum bertolonii as a potential agent for phytoremediation and phytomining of nickel. J Geochem Exp 59:75–86. doi:10.1016/S0375-6742(97)00010-1
Safari Sinegani AA, Khalilikhah F (2011) The effect of application time of mobilising agents on growth and phytoextraction of lead by Brassica napus from a calcareous mine soil. Environ Chem Lett 9:259–265. doi:10.1007/s10311-010-0275-1
Sahi SV, Israr M, Srivastava AK, Gardea-Torresdey JL, Parsons JG (2007) Accumulation, speciation and cellular localization of copper in Sesbania drummondii. Chemosphere 67:2257–2266. doi:10.1016/j.chemosphere.2006.12.006
Scrase-Field SAMG, Knight MR (2003) Calcium: just a chemical switch? Curr Opin Plant Biol 6:500–506. doi:10.1016/S1369-5266(03)00091-8
Van Nevel L, Mertens J, Oorts K, Verheyen K (2007) Phytoextraction of elements from soils: how far from practice? Environ Pollut 150:34–40. doi:10.1016/j.envpol.2007.05.024
Walker DJ, Bernal MP (2003) The effects of copper and lead on growth and zinc accumulation of Thlaspi caerulescens J. and C. Presl: implications for phytoremediation of contaminated soils. Water Air Soil Pollut 151:361–372. doi:10.1023/B:WATE.0000009901.89000.40
Wang X, Wang Y, Mahmood Q, Islam E, Jin X, Li T, Yang X, Liu D (2009) The effect of EDDS addition on the phytoextraction efficiency from Pb contaminated soil by Sedum alfredii Hance. J Hazard Mater 168:530–535. doi:10.1016/j.jhazmat.2009.02.074
Weatherall A, Proea MF, Craig J, Cameron AD, McKay HM, Midwood AJ (2006) Tracing N, K, Mg and Ca released from decomposing biomass to new tree growth. Part II: a model system simulating root decomposition on clearfell sites. Biomass Bioenerg 30:1060–1066. doi:10.1016/j.biombioe.2005.12.016
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511. doi:10.1093/aob/mcg164
Wilkins DA (1978) The measurement of tolerance to edaphic factors by means of root growth. New Phytol 80:623–633. doi:10.1111/j.1469-8137.1978.tb01595.x
Wójcik P (1998) Odżywianie się roślin wyższych wapniem. Calcium nutrition of higher plants. Wiadomości Botaniczne 42:41–52 (in Polish)
Acknowledgments
The authors wish to acknowledge the financial support of part of the studies by project grants N N305 372538 and N R12 0065 10, from the Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Kuzniak-Gebarowska.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Mleczek, M., Gąsecka, M., Drzewiecka, K. et al. Copper phytoextraction with willow (Salix viminalis L.) under various Ca/Mg ratios. Part 1. Copper accumulation and plant morphology changes. Acta Physiol Plant 35, 3251–3259 (2013). https://doi.org/10.1007/s11738-013-1360-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1360-4