Abstract
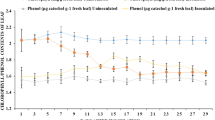
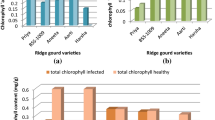
Ten genotypes of sugar beet plant either monogerm or multigerm seeds were screened under greenhouse conditions for both susceptibility and biochemical reaction to root-knot nematode (RKN) Meloidogyne incognita. All the tested genotypes were susceptible to nematode infection according to the number of root galls and gall indices. All infected genotypes exhibited significant reduction in chlorophyll a, b and carotenoids compared to non-infected ones. The total indole acetic acid and total phenolic compounds contents (mean of both shoot and root) increased significantly in most infected genotypes compared to non-infected genotypes except Disk-01-99 and Monte Rosa as well as LP16 and LP15 genotypes, respectively. Also, total polyamine contents (putrescine, spermidine and spermine) showed significant increases in response to infection with nematodes in all genotypes. The same trend was observed in lipid peroxidation expressed with malondialdehyde content in all tested genotypes. Activities of polyphenol oxidase, peroxidase, superoxide dismutase and catalase enzymes were also induced in most infected genotypes compared with non-infected genotypes. Generally, infection with RKNs induced the appearance of new protein bands at molecular masses 303, 288, 42 and 37 KDa in all infected genotypes. The differentiation in the appearance and/or disappearance of protein bands according to susceptibility to infection reflects the variation between genotypes in defense against infection.





Similar content being viewed by others
Abbreviations
- CAT:
-
Catalase
- H2O2 :
-
Hydrogen peroxide
- IAA:
-
Indole acetic acid
- MDA:
-
Malondialdehyde
- OD:
-
Optical density
- PAL:
-
Phenyl alanine ammonia lyase
- PDAB:
-
Para-dimethylamino-benzoic acid
- PA:
-
Polyamine
- POX:
-
Peroxidase
- PPO:
-
Polyphenol oxidase
- Put:
-
Putrescine
- RKNs:
-
Root-knot nematodes
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- Spd:
-
Spermidine
- Spm:
-
Spermine
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloro acetic acid
References
Abd El-Monem AA (2007) Polyamines as modulator of wheat growth, metabolism and productivity under high temperature stress. PhD thesis, Faculty of Science, Ain Shams University, Cairo, Egypt
Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784
Ayesh AM, Rowayshed GH, Deabes MM (2002) Inhibitory effect of some food additives on biogenic amines formation in beef sausages trials. Egypt J Nut 17:197–202
Berger S, Benediktyova Z, Matous K, Benfig K, Mueller MJ, Medbal L, Roitsch T (2007) Visualization of dynamics of plant–pathogen interaction by novel combination of chlorophyll fluorescence imaging and statistical analysis: differential effects of virulent and avirulent strains of P. syringae and of oxylipins on A. thaliana. J Exp Bot 58:797–806
Bhalerao RP, Eklof J, Ljung K, Marchant A, Bennett M, Sandberg G (2002) Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J 29:325–332
Bi JL, Felton GW (1995) Foliar oxidative stress and insect herbivory: primary compounds, secondary metabolites and reactive oxygen species as components of induced resistance. J Chem Ecol 21:1511–1530
Boot KJM, van Brussel AAN, Tak T, Spaink HP, Kijne JW (1999) Lipochitin oligosaccharides from Rhizobium leguminosarum bv. Viciae reduce auxin transport capacity in Vicia sativa subsp nigra roots. Mol Plant Microbe Interact 12:839–844
Bowler C, Slooten L, Vandenbranden S, De Rycke R, Botterman J, Sybesma C, Van Montagu M, Inzé D (1991) Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J 10:1723–1732
Cavalcanti FR, Resende ML, Lima SP, Silveira JA, Oliveira JT (2007) Activities of antioxidant enzymes and photosynthetic responses in tomato pre-treated by plant activators and inoculated by Xanthomonas vesicatoria. Physiol Mol Plant Pathol 68:198–208
Chaerle L, Leinonen I, Jones HG, Straeten DVD (2007) Monitoring and screening plant populations with combined thermal and chlorophyll fluorescence imaging. J Exp Bot 58:773–784
Chen JX, Wang XF (2006) Plant physiology experimental guide. Higher Education Press, Beijing, pp 24–25, 55–56
Chen S, Olbrich A, Heyser RL, Eberhard Fritz E, Polle A (2009) Quantitative X-ray microanalysis of hydrogen peroxide within plant cells. Microsc Res Tech 72:49–60
Danil AD, George CM (1972) Peach seed dormancy in relation to endogenous inhibitors and applied growth substances. J Am Soc Hortic Sci 17:621–624
Dewar AM, Cooke DA (2006) Pests. In: Draycott P (ed) Sugar beet. Blackwell Publishing Ltd, Oxford, pp 316–358
Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benkova E (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci USA 105:8790–8794
El-Khallal SM (2007) Induction and modulation of resistance in tomato plants against Fusarium wilt disease by bioagent fungi (arbuscular mycorrhiza) and/or hormonal elicitors (Jasmonic acid & Salicylic acid): 2-Changes in the antioxidant enzymes, phenolic compounds and pathogen related-proteins. Aust J Basic Appl Sci 1:717–732
Gómez Ros LV, Paradiso A, Gabaldón C, Pedreño MA, de Gara L, Ros Barceló A (2006) Two distinct cell sources of H2O2 in the lignifying Zinnia elegans cell culture system. Protoplasma 227:175–183
Grunewald W, Cannoot B, Friml J, Gheysen G (2009) Parasitic nematodes modulate PIN-mediated auxin transport to facilitate infection. PLoS Pathog 5:e1000266
Hirt H (2000) Connecting oxidative stress, auxin, and cell cycle regulation through a plant mitogen-activated protein kinase pathway [comment]. Proc Natl Acad Sci 97:2405–2407
Huang G, Gao B, Maier T, Allen R, Davis EL, Baum TJ, Hussey RS (2003) A profile of putative parasitism genes expressed in the oesophageal gland cells of the root-knot nematode Meloidogyne incognita. Mol Plant Microbe Interact 16:376–381
Jung S (2004) Variation in antioxidant metabolism of young and mature leaves of Arabidopsis thaliana subjected to drought. Plant Sci 166:459–466
Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux P (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9:627–640
Khattab H (2007) The defense mechanism of cabbage plant against phloem-sucking aphid (Brevicoryne brassicae L.). Aust J Basic Appl Sci 1:56–62
Kong FX, Hu W, Chao SY, Sang WL, Wang LS (1999) Physiological responses of mexicana to oxidative stress of SO2. Environ Exp Bot 42:201–209
Kumar KB, Khan PA (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Indian J Exp Bot 20:412–416
Larsen P, Harbo A, Klungron S, Ashein TA (1962) On the biosynthesis of some indole compounds in Acetobacter xylinum. Physiol Plant 15:552–565
Leng P, Su S, Wei F, Yu F, Duan Y (2009) Correlation between browning, total phenolic content, polyphenol oxidase and several antioxidation enzymes during pistachio tissue culture. Acta Hortic (ISHS) 829:127–132
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P (eds) Current protocols in food analytical chemistry (CPFA). John Wiley and Sons, New York, pp F4.3.1–F4.3.8
Mathesius U (2001) Flavonoids induced in cells undergoing nodule organogenesis in white clover are regulators of auxin breakdown by peroxidase. J Exp Bot 52:419–426
Mathesius U (2003) Conservation and divergence of signalling pathways between roots and soil microbes—the rhizobium–legume symbiosis compared to the development of lateral roots, mycorrhizal interactions and nematode-induced galls. Plant Soil 255:105–119
Mathesius U (2008) Auxin—at the root of nodule development? Funct Plant Biol 35:651–668
Mathesius U, Schlaman HRM, Spaink HP, Sautter C, Rolfe BG, Djordjevic MA (1998) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 14:23–34
Melo GA, Shimizu MM, Mazzafera P (2006) Polyphenoloxidase activity in coffee leaves and its role in resistance against the coffee leaf miner and coffee leaf rust. Phytochemistry 67:277–285
Mietz JL, Karmas E (1977) Chemical quality index of canned tuna as determined by high-pressure liquid chromatography. J Food Sci 42:155–158
Mithöfer A, Schulze B, Boland W (2004) Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Lett 566:1–5
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
MSTAT-C (1988) MSTAT-C, a microcomputer program for the design, arrangement and analysis of agronomic research. Michigan State University, East Lansing
Panella L, Lewellen RT (2007) Broadening the genetic base of sugar beet: introgression from wild relatives. Euphytica 154:383–400
Peer WA, Murphy AS (2007) Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci 12:556–563
Polidoros AN, Mylona PV, Scandalios JP (2001) Transgenic tobacco plants expressing the maize Cat2 gene have altered catalase levels that affect plant–pathogen interactions and resistance to oxidative stress. Transgenic Res 10:555–569
Predieri S, Norma MA, Krizek DT (1995) Influence of UV-B radiation on membrane lipid composition and ethylene of evolution in ‘Doyenne d’Hiver’ pear shoots grown in vitro under different photosynthetic photo fluxes. Environ Exp Bot 35:152–260
Rani CI, Veeraragavathatham D, Sanjutha S (2008) Analysis on biochemical basis of root-knot nematode (Meloidogyne incognita) resistance in tomato (Lycopersicon esculentum Mill.) Res. J Agric Biol Sci 4:866–870
Reuveni R, Shimoni M, Karchi Z, Kuc J (1992) Peroxidase activity as a biochemical marker for resistance of muskmelon on (Cucumis meb) to Pseudoperonospora cubensis. Phytopathology 82:749–753
Rhee HJ, Kim EJ, Lee JK (2007) Physiological polyamines: simple primordial stress molecules. J Cell Mol Med 11:685–703
Rivero RM, Ruiz JM, Garcia PC, Lopez – Lefebre LR, Sanchy E, Romero L (2001) Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and water melon plants. Plant Sci 160:315–321
Robert-Seilaniantz A, Navarro L, Bari R, Jones JDG (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10:372–379
Sasser JN, Carter C, Hartman KM (1984) Standardization of host suitability studies and reporting of resistance to root-knot nematodes. A Cooperative Publication of the Department of Plant Pathology North Carolina State University and U.S. Agency for International Development. Raleigh, North Carolina, USA, p 7
Sheri LH, Ncolas ES, Michae TK, Joanna BG (2000) Comparison of protein expressed by Pseudomonas aeruginosa strains representing initial and chronic isolates from a cystic fibrosis patient: an analysis by 2-D gel electrophoresis and capillary column liquid chromatograph tandem mass spectrometry. Microbiology 146:2495–2508
Soliva RC, Elez P, Sebastián M, Martín O (2001) Evaluation of browning effect on avocado purée preserved by combined methods. Innov Food Sci Emerg Technol 1:261–268
Spoel HS, Dong X (2008) Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 3:348–351
Torres MA, DG Jonathan DG, Dangl JL (2006) Reactive oxygen species signaling in response to pathogen. Plant Physiol 141:373–378
Van Noorden GE, Kerim T, Goffard N, Wiblin R, Pellerone FI, Rolfe BG, Mathesius U (2007) Overlap of proteome changes in Medicago truncatula in response to auxin and Sinorhizobium meliloti. Plant Physiol 144:1115–1131
Velazhahan R, Muthukrishnan S (2004) Transgenic tobacco plants constitutively overexpressing a rice thaumotin-like protein (PR-5) show enhanced resistance to Alternaria alternata. Biol Plant 47:347–354
Walters DR (2003) Polyamines and plant disease. Phytochemistry 64:97–107
Wang T, Quisenberry SS, Ni X, Tolmay V (2004) Enzymatic chlorophyll degradation in wheat near-isogenic lines elicited by cereal aphid (Homoptera: Aphididae) feeding. J Econ Entomol 97:661–667
Wang XH, Replogle A, Davis EL, Mitchum MG (2007) The tobacco cel7 gene promoter is auxin-responsive and locally induced in nematode feeding sites of heterologous plants. Mol Plant Pathol 8:423–436
Wasson AP, Ramsay K, Jones MG, Mathesius U (2009) Differing requirements for flavonoids during the formation of lateral roots, nodules and root-knot nematode galls in Medicago truncatula. New Phytol 183:167–179
Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, van Montagu M, Inzé D, van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J J16:4806–4816
Zhang S, Klessig DF (2000) In: Hirt H (ed) MAP kinases in plant signal transduction. Springer, Heidelberg, pp 65–84
Zhou YH, Yu JQ, Mao WH, Huang LF, Song XS, Nogués S (2006) Genotypic variation on Rubisco expression, photosynthetic electron flow and antioxidant metabolism in the chloroplasts of chill-exposed cucumber plants. Plant Cell Physiol 47:192–199
Acknowledgments
This work was funded by The National Research Centre through the project entitled “The development of integrated management to improve productivity (quantity and quality) of sugar beet”. Project No. 8040716 during 2007–2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Barna.
Rights and permissions
About this article
Cite this article
Korayem, A.M., El-Bassiouny, H.M.S., Abd El-Monem, A.A. et al. Physiological and biochemical changes in different sugar beet genotypes infected with root-knot nematode. Acta Physiol Plant 34, 1847–1861 (2012). https://doi.org/10.1007/s11738-012-0983-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-0983-1