Abstract:
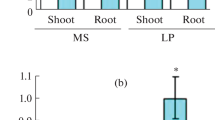
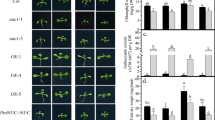
MAX4 gene has been shown to be involved in the regulation of shoot branching in Arabidopsis (Arabidopsis thaliana). However, little is known about the role of MAX4 gene in low inorganic phosphate (Pi) stress response in Arabidopsis. Here we showed that MAX4 gene is involved in the regulation of low Pi stress response in Arabidopsis. MAX4 gene was repressed by low Pi stress, and the max4 mutants showed lower anthocyanin content and longer primary root length. In addition, max4 mutant plants also displayed altered root architecture such as increased root-to-shoot ratio, lower lateral root number and root hair density compared with wild-type plants under low Pi stress. Higher total Pi contents were detected in shoots and roots of max4 plants than those of wild-type plants when subjected to low Pi stress, which was associated, at least in part, with increase in expression of WRKY75 as well as AtPT1 and AtPT2 genes encoding high-affinity Pi transporters. Taken together, all these results suggest that MAX4 gene mediates low Pi stress response, at least in part, by regulating the expression of WRKY75 as well as AtPT1 and AtPT2 genes.





Similar content being viewed by others
Abbreviations
- AtACP5 :
-
Acid phosphatase 5
- AtIPS1 :
-
Induced by phosphate starvation 1
- AtPHR1 :
-
Phosphate starvation response 1
- AtPT1 :
-
Arabidopsis thaliana phosphate transporter 1
- AtPT2 :
-
Arabidopsis thaliana phosphate transporter 2
- AtRNS1 :
-
Ribonuclease 1
- AtSIZ1 :
-
Arabidopsis SUMO E3 ligase sap and miz1
- MAX4 :
-
More axillary branching 4
- MS:
-
Murashige and Skoog
- Pi:
-
Inorganic phosphate
- QPCR:
-
Quantitative real-time PCR
- RT:
-
Reverse transcription
- WRKY75 :
-
Wrky DNA-binding protein 75
- WT:
-
Wild-type
References
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Ames BN (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8:115–118
Cao SQ, Chen ZY, Liu GQ, Jiang L, Yuan HB, Ren G, Bian XH, Jian HY, Ma XL (2009) The Arabidopsis Ethylene-Insensitive 2 gene is required for lead resistance. Plant Physiol Biochem 47:308–312
Chen ZH, Nimmo GA, Jenkins GI, Nimmo HG (2007) BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis. Biochem J 405:191–198
Chen YF, Li LQ, Xu Q, Kong YH, Wang H, Wu WH (2009) The WRKY6 transcription factor modulates PHOSPHATE1 expression in response to low Pi stress in Arabidopsis. Plant Cell 21:3554–3566
Devaiah BN, Karthikeyan AS, Raghothama KG (2007a) WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol 143:1789–1801
Devaiah BN, Nagarajna VK, Raghothama KG (2007b) Phosphate homeostasis and root development in Arabidopsis are synchronized by the zing finger transcription factor ZAT6. Plant Physiol 145:147–159
Devaiah BN, Madhuvanthi R, Karthikeyan AS, Raghothama KG (2009) Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol Plant 2:43–58
Franco-Zorilla JM, González E, Bustos R, Linhares F, Leyva A, Paz-Ares J (2004) The transcriptional control of plant responses to phosphate limitation. J Exp Bot 55:285–293
Hammond JP, Bennett MJ, Bowen HC, Broadley MR, Eastwood DC, May ST, Rahn C, Swarup R, Woolaway KE, White PJ (2003) Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiol 132:578–596
Hernández G, Ramírez M, Valdés-López O, Tesfaye M, Graham MA, Czechowski T, Schlereth A, Wandrey M, Erban A, Cheung F (2007) Phosphorus stress in common bean: root transcript and metabolic responses. Plant Physiol 144:752–767
Jain A, Poling MD, Karthikeyan AS, Blakeslee JJ, Peer WA, Titapiwatanakun B, Murphy AS, Raghothama KG (2007) Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiol 144:232–247
Kim J, Yi H, Choi G, Shin B, Song PS, Choi G (2003) Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell 15:2399–2407
Lipton DS, Blanchar RW, Blevins DG (1987) Citrate, malate and succinate concentration in exudates from P-sufficient and P-stressed Medicago sativa L seedlings. Plant Physiol 85:315–317
López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol 6:280–287
Lynch JP, Brown KM (2001) Topsoil foraging: an architectural adaptation of plants to low phosphorus availability. Plant Soil 237:225–237
Ma Z, Baskin TI, Brown KM, Lynch JP (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol 131:1381–1390
Misson J, Raghothama KG, Jain A (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci USA 102:11934–11939
Miura K, Lee J, Jin JB, Yoo CY, Miura T, Hasegawa PM (2009) Sumoylation of ABI5 by the Arabidopsis SUMO E3 ligase SIZ1 negatively regulates abscisic acid signaling. Proc Natl Acad Sci USA 106:5418–5423
Muchhal US, Pardo JM, Raghothama KG (1996) Phosphate transporters from the higher plant Arabidopsis thaliana. Proc Natl Acad Sci USA 93:10519–10523
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Pant BD, Buhtz A, Kehr J, Scheible WR (2008) MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J 53:731–773
Pérez-Torres CA, López-Bucio J, Cruz-Ramírez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, Herrera-Estrella L (2008) Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20:3258–3272
Raghothama KG (2000) Phosphate transport and signaling. Curr Opin Plant Biol 3:182–187
Rouached H, Arpat AB, Poirier Y (2010) Regulation of phosphate starvation responses in plants: signaling players and cross-talks. Mol Plant 3(2):288–299
Rubio V, Linhares F, Solano R, Martín AC, Iglesias J, Leyva A, Paz-Ares J (2001) A conserved MYB transcription factor involved in phosphate starvation signalling both in vascular plants and in unicellular algae. Genes Dev 15:2122–2133
Sánchez-Calderón L, López-Bucio J, Chacón-López A, Gutiérrez-Ortega A, Hernández-Abreu E, Herrera-Estrella L (2006) Characterization of low phosphorus insensitive mutants reveals a crosstalk between low phosphorus-induced determinate root development and the activation of genes involved in the adaptation of Arabidopsis to phosphorus deficiency. Plant Physiol 140:879–889
Schikora A, Schmidt W (2001) Acclimative changes in root epidermal cell fate in response to Fe and P deficiency: a specific role for auxins? Protoplasma 218:67–75
Shin H, Shin HS, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J 39:629–642
Sorefan K, Booker J, Haurogné K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, Leyser O (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17:1469–1474
Uhde-Stone C, Zinn KE, Ramirez-Yáñez M, Li A, Vance CP, Allan DL (2003) Nylon filter arrays reveal differential gene expression in proteoid roots of white lupin in response to phosphorus deficiency. Plant Physiol 131:1064–1079
Wang Y, Ribot C, Rezzonico E, Poirier Y (2004) Structure and expression profile of the Arabidopsis PHO1 gene family indicates a broad role in inorganic phosphate homeostasis. Plant Physiol 135:400–411
Wu P, Ma L, Hou X (2003) Phosphate starvation triggers distinct alterations of genome expression in Arabidopsis roots and leaves. Plant Physiol 132:1260–1271
Yang XJ, Finnegan PM (2010) Regulation of phosphate starvation responses in higher plants. Ann Bot doi:10.1093/aob/mcq015
Yi KK, Wu ZC, Zhou J, Du LM, Guo LB, Wu YR, Wu P (2005) OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiol 138:2087–2096
Yuan JS, Reed A, Chen F, Stewart CN (2006) Statistical analysis of real-time PCR data. BMC Bioinformatics 7:85
Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136:2621–2632
Acknowledgments
We would like to thank Zhenyi Chen, Xiaohui Bian, Shengchao Lv and Weiwei Meng for their technical assistances. This work was supported by the National Natural Science Foundation of China (grant no. 20777014), the National Transgenic Plant R&D Project of China (2009ZX08009-063B), the Great Project of Natural Science Foundation at Anhui Provincial Education Department (KJ2010ZD04), and the Funds for Creative Research Groups of Hefei University of Technology (2009HGCX0233).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.-H. Liu.
Rights and permissions
About this article
Cite this article
Jiang, L., Jian, H., Qian, J. et al. MAX4 gene is involved in the regulation of low inorganic phosphate stress responses in Arabidopsis thaliana. Acta Physiol Plant 33, 867–875 (2011). https://doi.org/10.1007/s11738-010-0612-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0612-9