Abstract
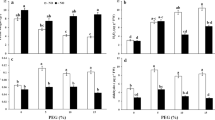
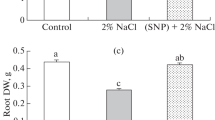
Osmotic stress associated with drought and salinity is a serious problem that inhibits the growth of plants mainly due to disturbance of the balance between production of ROS and antioxidant defense and causes oxidative stress. In this research, sodium nitroprusside (SNP) was used as NO donor in control and drought-stressed plants, and the role of NO in reduction of oxidative damages were investigated. In this study, we observed that SNP pretreatment prevented drought-induced decrease in RWC and membrane stability index, increase in lipid peroxidation and lipoxygenase activity and increase in hydrogen peroxide content. However, pretreatment of plants with SNP and phenyl 4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (a NO scavenger) reversed the protective effects of SNP suggesting that protective effect by SNP is attributable to NO release. In addition, the relationship between these defense mechanisms and activity of antioxidant enzymes were checked. Results showed that in drought-stressed plants ascorbate peroxidase (APX), guaiacol peroxidase (GPX) and catalase activities were elevated over the controls, while GR decreased under drought condition. Activity of GPX was inhibited under SNP pretreatment in drought-stressed plants specially, while the activity of APX and GR increased under SNP pretreatment and it seems that under this condition APX had a key role of detoxification of ROS in tomato plants. This result corresponded well with ASA and total acid-soluble thiols content. Therefore, reduction of drought-induced oxidative damages by NO in tomato leaves is most likely mediated through either NO ability to scavenge active oxygen species or stimulation of antioxidant enzyme such as APX.



Similar content being viewed by others
References
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344. doi:10.1046/j.1365-3040.2001.00778.x
Arasimowicz M, Floryszak-Wieczorek J (2007) Nitric oxide as a bioactive signaling molecule in plant stress responses. Plant Sci 172:876–887. doi:10.1016/j.plantsci.2007.02.005
Beligni MV, Lamatina L (1999) Nitric oxide counteract cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 208:337–344. doi:10.1007/s004250050567
Blatt MR (2002) Ca2+ signalling and control of guard-cell volume in stomatal movement. Curr Opin Plant Biol 3:196–204
Bohner HJ, Jensen RG (1996) Strategies for engineering water-stress tolerance in plants. Trends Biotechnol 14:89–97. doi:10.1016/0167-7799(96)80929-2
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Cheng FY, Hsu SY, Kao CH (2002) Nitric oxide counteract the senescence of detached rice leaves induced by dehydration and polyethylene glycol but not by sorbitol. Plant Growth Regul 38:265–272. doi:10.1023/A:1021529204978
Clarck D, Dunar J, Navarre DA, Klessig DF (2000) Nitric oxide inhibition of tobacco catalase and ascorbate peroxidase. Mol Plant Microb Interact 14:1380–1384. doi:10.1094/MPMI.2000.13.12.1380
de Pinto MC, Francis D, De Gara L (1999) The redox state of the ascorbate–dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 209:90–95
Del Rio LA, Corpas FJ, Barroso JB (2004) Nitric oxide and nitric oxide synthase activity in plants. Phytochemistry 65:783–792. doi:10.1016/j.phytochem.2004.02.001
Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interaction between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Plant Boil 98:13454–13459
Dhindsa RS, Dhindsa P, Thorpe AT (1981) Leaf senescence correlated with increased levels of membrane permeability and lipid peroxidation and decrease levels of superoxide dismutase and catalase. J Exp Bot 32:93–101. doi:10.1093/jxb/32.1.93
Doderer A, Kokkelink I, Vanderween S, Valk B, Schrom AW, Douma AC (1992) Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta 1120:97–104
Duan X, Su X, You Y, Qu H, Li Y, Jiang Y (2007) Effects of nitric oxide on pericarp browning of harvested longan fruit in relation to phenolic metabolism. Food Chem 104:571–576. doi:10.1016/j.foodchem.2006.12.007
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplast: a proposed role in ascorbic acid metabolism. Planta 133:21–25. doi:10.1007/BF00386001
Gl Ellman (1959) Tissue sulfydryl groups. Arch Biochem Biophys 82:70–77. doi:10.1016/0003-9861(59)90090-6
Halliwel B, Gutteridge JM (1984) Oxygen toxicity, oxygen radicals, transition metal and disease. Biol chem J 219:1–14
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast, kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. doi:10.1016/0003-9861(68)90654-1
Hsu YT, Kao CH (2004) Cd toxicity is reduced by nitric oxide in rice leaves. Plant Growth Regul 42:227–238. doi:10.1023/B:GROW.0000026514.98385.5c
Kopka J, Provart JN, Muller B (1997) Potato guard cells respond to drying soil by a complex change in the expression of genes related to carbon metabolism and turgor regulation. Plant J 11:871–888
Kopyra M, Gwozdz EA (2003) Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant Physiol Biochem 41:1011–1017. doi:10.1016/j.plaphy.2003.09.003
Laspina NV, Groppa MD, Tomaro ML, Benavides MP (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323–330. doi:10.1016/j.plantsci.2005.02.007
Madhusudhan R, Ishikawa T, Sawa Y, Shiqeoka S, Shibata H (2003) Characterization of an ascorbate peroxidase in plastids of tobacco BY-2 cells. Physiol Plant 117:550–557. doi:10.1034/j.1399-3054.2003.00066.x
Mata CG, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126:1196–1204. doi:10.1104/pp.126.3.1196
Meirs P, Hada S, Aharoni N (1992) Ethylene increased accumulation of fluorescent lipid peroxidation products detected during senescence of parsley by a newly developed method. J Am Soc Hortic Sci 117:128–132
Muir SR, Sanders D (1996) Pharmacology of Ca2+ release from red beet microsomes suggests the present of ryanodine receptor homologs in higher plants. FEBS Let 395:39–42
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Neill S, Desikan R, Clarke A, Hancock J (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128:13–16
Neill S, Desikan R, Hancock J (2003a) Nitric oxide as a mediator of ABA signaling in stomatal guard cells. Bulgar J Plant Physiol Special Issue pp 124–132
Neill S, Desikan R, Hancock J (2003b) Nitric oxide signaling in plant. New Phytol 159:11–35
Sairam RK, Deshmukh PS, Shukla DS (1997) Tolerance to drought and temperature stress is relation to increased antioxidant enzyme activity in Wheat. J Agron Crop Sci 178:171–177
Scandadalios JG (1993) Oxygen stress and superoxide dismutase. Plant Physiol 101:7–12
Shi Q, Ding F, Wang X, Wei M (2007) Exogenous nitric oxide protects cucumber roots against oxidative stress induced by salt stress. Plant Physiol Biochem 45:542–550
Shinozaki K, Yamaguchi K (1997) Gen expression and signal transduction in water-stress response. Plant Physiol 115:327–334
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Wheatherley PE (1950) Studies in water relations of cotton plants. The field measurement of water deficit in leaves. New Phytol 49:81–87
Wu Y, Kuzma J, Marechal E, Graeff R, Lee HC, Foster R, Chua N (1997) Abscisic acid signaling through cyclic ADP-ribose in plants. Science 282:226–230
Zhang J, Kirkham MB (1995) Water relations of water-stressed, split-root C4 (Sorghum bicolor; Poaceae) and C3 (Helianthus annus; Asteraceae) plants. Am J Bot 82:1220–1229
Zhang Z, Pang X, Duan X, Ji ZL, Jiang Y (2005) Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem 90:47–52
Zhao Z, Chen g, Zhang C (2001) Interaction between reactive oxygen species and nitric oxide in drought-induced abscisic acid synthesis in root tips of wheat seedlings. Aust J Plant Physiol 28:1055–1061
Zhu S, Liu M, Zhou J (2006) Inhibition by nitric oxide of ethylene biosynthesis and lipoxygenase activity in peach fruit during storage. Postharvest Biol Technol 42:41–48
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Bartosz.
Rights and permissions
About this article
Cite this article
Nasibi, F., Kalantari, K.M. Influence of nitric oxide in protection of tomato seedling against oxidative stress induced by osmotic stress. Acta Physiol Plant 31, 1037–1044 (2009). https://doi.org/10.1007/s11738-009-0323-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-009-0323-2