Abstract
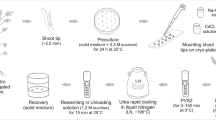
Epilobium angustifolium L. (fireweed) is a medicinal plant that has been used to treat diarrhea, mucous colitis, irritable-bowel syndrome, skin problems, prostate problems, menstrual disorders, asthma, whooping cough, and hiccups. A highly efficient and rapid regeneration system via multiple shoot formation was developed for fireweed. Explants (leaf, petiole, root, and stem segments) excised from sterile seedlings were cultured on medium supplemented with different concentrations and combinations of various plant growth regulators. Explant browning, a major problem for regeneration, was overcome by adding 100 mg/l ascorbic acid to all prepared media containing growth regulator combinations. Root explants formed more shoots than other explants. Best shoot proliferation was obtained from root explants cultured on media with 0.1 mg/l BA and 0.5 mg/l IAA. Regenerated shoots were transferred to rooting media containing different concentrations of IAA, IBA, NAA or 2,4-D. Most shoots developed roots on medium with 0.5 mg/l IAA. Rooted explants were transferred to vermiculate in Magenta containers for acclimatization and after 3 weeks they were planted in to plastic pots containing potting soil and maintained in the plant growth room.

Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- BA:
-
Benzyladenine
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- KIN:
-
Kinetin
- MSMO:
-
Murashige and Skoog’s minimal organics
- NAA:
-
Naphthalene acetic acid
- TDZ:
-
Thidiazuron
References
Akbudak MA, Babaoglu M (2005) Callus induction in small flowered willow herb (Epilobium parviflorum Schreb). J Plant Biochem Biot 14:189–191
Battinelli L, Tita B, Evandri MG, Mazzanti G (2001) Antimicrobial activity of Epilobium spp. Extracts. IL Farmaco 56:345–348
Baytop T (1999) Türkiye’de Bitkiler ile Tedavi. Nobel Tıp Kitabevleri, Istanbul, p 374
Broderick DH (1990) The biology of Canadian weeds. 93. Epilobium angustifolium L. (Onagraceae). Can J Plant Sci 70:247–259
Chevallier A (1996) The encyclopedia of medicinal plants. Dorling Kindersley Limited, London, p 185
Davis PH (1972) Flora of Turkey and the East Aegean Islands, vol 4. Edinburgh Univ. Press, Edinburgh, p 185
De Gyves EM, Sparks CA, Fieldsend AF, Lazzeri PA, Jones HD (2001) High frequency of adventitious shoot regeneration from commercial cultivars of evening primrose (Oenothera spp.) using thidiazuron. Ann Appl Biol 138:329–332
Dobelis IN (1990) Magic and medicine of plants. The Reader’s Digest Association, New York, p 185
Grieve M (1982) A modern herbal, vol 2. Dover Publications, New York, p 847
Hiermann A, Bucar F (1997) Studies of Epilobium angustifolium extracts on growth of accessory sexual organs in rats. J Ethnopharmacol 55:179–183
Hiermann A, Juan H, Sametz W (1986) Influence of Epilobium extracts on prostaglandin biosynthesis and carrageenin induced oedema of the rat paw. J Ethnopharmacol 17:161–169
Hiermann A, Reidlinger M, Juan H, Sametz W (1991) Isolation of the antiphlogistic principle from Epilobium angustifolium. Planta Med 57:357–360
Juan H, Sametz W, Hiermann A (1988) Anti-inflammatory effects of a substance extracted from Epilobium angustifolium. Agents Actions 23:106–107
Kilic A, Kilic A, Inandi T (2001) Folkloric treatment of tinea capitis with Epilobium angustifolium. Plast Reconstr Surg 108:1824–1825
Kiss A, Kowalski J, Melzig MF (2006a) Induction of neutral endopeptidase activity in PC-3 cells by an aqueous extract of Epilobium angustifolium L. and oenothein B. Phytomedicine 13:284–289
Kiss A, Kowalski J, Melzig MF (2006b) Effect of Epilobium angustifolium L. extracts and polyphenols on cell proliferation and neutral endopeptidase activity in selected cell lines. Pharmazie 61:66–69
Kuchuk N, Herrmann RG, Koop HU (1998) Plant regeneration from leaf protoplasts of evening primrose (Oenothera hookeri). Plant Cell Rep 17:601–604
Lombardi SP, Passos IRS, Nogueira MCS, Glória BA (2007) In vitro shoot regeneration from roots and leaf discs of Passiflora cincinnata Mast. Braz Arch Biol Technol 50:239–247
McColl J (2002) Willowherb (Epilobium angustifolium L.): biology, chemistry, bioactivity and uses. Agro Food Ind Hi Tec 13:18–22
Mehra-Palta A, Koop HU, Goes S, Troidl EM, Nagy G, Tyagi S, Kofer W, Herrmann RG (1998) Tissue culture of wild-type, interspecific genome/plastome hybrids and plastome mutants of evening primrose (Oenothera): controlled morphogenesis and transformation. Plant Cell Rep 17:605–611
Murashige T (1974) Plant propagation through tissue culture. Biologist 21:87–93
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Pati PK, Rath SP, Sharma M, Sood A, Ahuja PS (2006) In vitro propagation of rose—a review. Biotechnol Adv 24:94–114
Pavingerova D, Galis I, Ondrej M (1996) Tissue culture and transformation of Oenothera biennis. Biol Plantarum 38:27–32
Rauha JP, Remes S, Heinonen M, Hopia A, Kähkönen M, Kujala T, Pihlaja K, Vuorela H, Vuorela P (2000) Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol 56:3–12
Rout GR, Samantaray S, Das P (2000) In vitro manipulation and propagation of medicinal plants. Biotechnol Adv 18:91–120
Rout GR, Samantaray S, Mottleyi J, Das P (1999) Biotechnology of the rose: a review of recent progress. Sci Hortic 81:201–228
SAS Institute Inc (2002) SAS software for statistical analysis, version 8.0. North Carolina, USA
Shahzad A, Faisal M, Anis M (2007) Micropropagation through excised root culture of Clitoria ternatea and comparison between in vitro–regenerated plants and seedlings. Ann Appl Biol 150:341–349
Shikov AN, Poltanov EA, Dorman HJD, Makarov VG, Tikhonov VP, Hiltunen R (2006) Chemical composition and in vitro antioxidant evaluation of commercial water-soluble willow herb (Epilobium angustifolium L.) extracts. J Agric Food Chem 54:3617–3624
Slacanin I, Marston A, Hostettmann K, Delabays N, Darbellay C (1991) Isolation and determination of flavonol glycosides from Epilobium species. J Chromatogr A 557:391–398
Skrzypczak L, Skrzypczak-Pietraszek E, Lamer-Zarawska, Hojden B (1994) Micropropagation of Oenothera biennis L. and an assay of fatty acids. Acta Soc Bot Pol 63:73–177
Thiem B, Skrzypczak L, Lamer-Zarawska E (1999) Micropropagation of selected Oenothera species and preliminary studies on their secondary metabolites. Acta Soc Bot Pol 68:15–20
Tita B, Abdel-Haq H, Vitalone A, Mazzanti G, Saso L (2001) Analgesic properties of Epilobium angustifolium, evaluated by the hot plate test and the writhing test. IL Farmaco 56:341–343
Trigiano RN, Gray DJ (2000) Plant tissue culture: concepts and laboratory exercises, 2nd edn. CRC Press, Boca Raton
Vitalone A, Bordi F, Baldazzi C, Mazzanti G, Saso L, Tita B (2001) Anti-proliferative effect on a prostatic epithelial cell line (PZ-HPV-7) by Epilobium angustifolium L. IL Farmaco 56:483–489
Vitalone A, Guizzetti M, Costa LG, Tita B (2003a) Extracts of various species of Epilobium inhibit proliferation of human prostate cells. J Pharm Pharmacol 55:683–690
Vitalone A, McColl J, Thome D, Costa LG, Tita B (2003b) Characterization of the effect of Epilobium extracts on human cell proliferation. Pharmacology 69:79–87
Wichtl M (2004) Herbal drugs and phytopharmaceuticals. 3rd edn. Medpharm Scientific Publishers, CRC press, Stuttgart, p 191
Xiao XG, Branchard M (1995) In vitro high frequency plant regeneration from hypocotyl and root segments of spinach by organogenesis. Plant Cell Tissue Organ 42:239–244
Yucesan B, Turker AU, Gurel E (2007) TDZ-induced high frequency plant regeneration through multiple shoot formation in witloof chicory (Cichorium intybus L.). Plant Cell Tissue Organ 91:243–250
Zaid A (1984) In vitro browning of tissues and media with special emphasis to date palm cultures. Date Palm J 3:269–275
Zobayed SMA, Saxena PK (2003) In vitro-grown roots: a superior explant for prolific shoot regeneration of St. John’s wort (Hypericum perforatum L. cv ‘New Stem’) in a temporary immersion bioreactor. Plant Sci 165:463–470
Acknowledgments
The authors are grateful to The Scientific and Technological Research Council of Turkey (TUBITAK) for financial support [TBAG-2278(103T024) and TBAG-HD/27(105T040)]. We are grateful to Dr. Hakan Turker for his technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Lojkowska.
Rights and permissions
About this article
Cite this article
Turker, A.U., Mutlu, E.C. & Yıldırım, A.B. Efficient in vitro regeneration of fireweed, a medicinal plant. Acta Physiol Plant 30, 421–426 (2008). https://doi.org/10.1007/s11738-008-0136-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-008-0136-8