Abstract
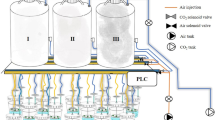
The effect of controlled carbon dioxide environment on in vitro shoot growth and multiplication in Feronia limonia (a tropical fruit plant, Family- Rutaceae) was studied. Carbon dioxide available in the ambient air of the growth room was insufficient for in vitro growth of the shoots alone. Also, the presence of sucrose only as the C-source in the medium (without CO2), was found to be inadequate for sustainable growth and multiplication of shoots. The carbon dioxide enrichment promoted shoot multiplication and overall growth. The promotory effect of CO2 was independent of the presence of sucrose in the medium. In the presence of both CO2 and sucrose, an additive effect was observed producing maximum shoot growth. In the absence of sucrose a higher concentration of CO2 (10.0)g m−3 was required to achieve photoautotrophic shoot multiplication comparable to ambient air controls. Highest leaf area per shoot cluster promoting shoot growth and multiplication was recorded under this treatment. Shoots growing on sucrose containing medium under controlled CO2 environment of 0.6 g m−3 concentration evoked better response than ambient air controls (shoots growing on sucrose containing medium) in growth room. This treatment produced the overall best response. The present study highlighted the possibility of photoautotrophic multiplication which might prove useful for successful hardening and acclimatization in tissue culture plants.
Similar content being viewed by others
Abbreviations
- BA:
-
6-benzyladenine
- Kn:
-
Kinetin
- NAA:
-
α-Napthalene acetic acid
- KHCO3 :
-
potassium bicarbonate
- K2CO3 :
-
potassium carbonate
- KOH:
-
potassium hydroxide
- MS:
-
Murashige and Skoog
- Na2CO3 :
-
sodium carbonate
- NaHCO3 :
-
sodium bicarbonate
- SM:
-
standard multiplication medium
- SCSM:
-
sucrose containing standard multiplication medium
- SFSM:
-
sucrose-free standard multiplication medium
References
Aitken-Christie, J., Davies, H., Kubota, C. and Kozai, T. 1990. Benefits of using a microporous membrane in tissue culture vessel lid for the micropropagation of Pinus radiata. — In: Abstr. VII Intl. Congress on Plant Tissue and Cell Culture, Amsterdam, The Netherlands, 86.
Cure, J.D., Rufty, T.W. Jr., and Israel, D. W. 1989. Alterations in soybean leaf development and photosynthesis in a CO2 enriched atmosphere. Bot. Gaz. 150: 337–345.
Dave, N. and Purohit, S. D. 2004. In vitro growth and shoot multiplication of Achras zapota in a controlled carbon dioxide environment. Biol. Plant. 48: 621–624.
De Proft, M.P., Maene, L.J. and Debergh, P.C. 1985. Carbon dioxide and ethylene evolution in culture atmosphere of Magnolia cultured in vitro. Physiol. Plant. 65: 375–379.
Deng, R. and Donnelly, D.J. 1993. In vitro hardening of red raspberry through CO2 enrichment and relative humidity reduction on sugar-free medium. Can. J. Plant. Sci. 73: 1105–1113.
Doi, M., Oda, H. and Asahira, T. 1989. In vitro atmosphere of cultured C3 and CAM plants in relation to day-lengths. Environ. Control. Biol. 27: 9–13.
During, H. and Harst, M. 1996. Stomatal behaviour, photosynthesis and photorespiration of in vitro grown grapevines: Effects of light and CO2. Vitis 35: 163–167.
Fujiwara, K., Kozai, T. and Watanabe, I. 1987. Fundamental studies on environments in plant tissue culture vessels.(3) Measurements of carbon dioxide gas concentration in closed vessels containing tissue cultured plantlets and estimates of Net Photosynthetic Rates of the Plantlets. J. Agr. Meteorol. 43: 21–30.
Jeong, B.R., Fujiwara, K. and Kozai, T. 1993. Carbon dioxide enrichment in autotrophic micropropagation: Methods and advantages. Hort. Technol. 3: 332–334.
Jeong, B. R., Fujiwara, K. Kozai, T. 1995. Environmental control and photoautotrophic micropropagation. Hort. Rev. 17: 127–172.
Kirdmanee, C., Kitaya, Y. and Kozai, T. 1995. Effect of CO2 enrichment and supporting material in vitro on photoautotrophic growth of Eucalyptus plantlets in vitro and ex vitro. In Vitro Cell. Dev. Biol. Plant 31: 144–149.
Kirtikar, K. R.; Basu, B. D. 1993. Indian Medicinal Plants, Vol. I, Orient Enterprises, Dehradun, India, 496–498.
Kohlmaier, G.H., Sire, E.O. and Janecek, A. 1989. Modelling the seasonal contribution of a CO2 fertilization effect of the terresterial vegetation to the amplitude increase in atmospheric CO2 at Mauna Loa Observatory. Tellus 41:487–510.
Kozai, T. and Iwanami, Y. 1988. Effect of CO2 enrichment and sucrose concentration under high photon fluxes on plantlet growth of carnation [Dianthus caryophyllus (L.)] in tissue culture during preparation stage. J. Jap. Soc. Hort. Sci. 57: 279–288.
Kozai, T., Fujiwara, K., Hayashi, M. and Aitken-Christie, J. 1992. The in vitro environment and its control in micropropagation. In: K. Kurata, and T. Kozai, (Eds.) Transplant Production Systems. Kluwer Academic Publishers, Dordrecht, The Netherlands, 247–282.
Langford, P.J. and Wainwright, H. 1987. Effects of sucrose concentration on the photosynthetic ability of rose shoots in vitro. Ann. Bot. 60: 633–640.
Lemos, E.E.P. and Blake, J. 1996. Control of leaf abscission in nodal cultures of Annona squamosa L. J. Hort. Sci. 71: 721–728.
Mousseau, M. 1986. CO2 enrichment in vitro: Effect on autotrophic and heterotrophic cultures of Nicotiana tabacum. Photosynthesis Research 8: 187–191.
Murashige T. and Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant 15: 473–497.
Nowak, J. and Shulaev, V. 2003. Priming for transplant stress resistance in in vitro propagation. In Vitro Cell Dev. Biol. Plant. 39: 107–124.
Rogers, H.H., Runion, G.B. and Krupa, S.V. 1994. Plant responses to atmospheric CO2 enrichment with emphasis on roots and the rhizosphere. Environ. Pollution 83: 155–189.
Solarova, J. and Pospisilova, J. 1997. Effect of carbon dioxide enrichment during in vitro cultivation and acclimation to ex vitro conditions. Biol. Plant. 39: 23–30.
Solarova, J., Souckova, D., Ullmann, D. and Pospisilova, J. 1996. In vitro culture: environmental conditions and plantlet growth as affected by vessel and stopper types. Hort. Sci. (Prague) 23: 51–58.
Tak, K. 1993. Tissue culture studies on some forest tree species of Aravallis in south Rajasthan. Ph. D. Thesis, M.L. Sukhadia University, Udaipur, India.
Thompson, M.R. and Thorpe, T.A. 1987. Metabolic and non- metabolic roles of carbohydrates. In: J.M. Bonga and D.J. Durzan (Eds.), Cell and Tissue Culture in Forestry. Martinus Nijhoff, Dordrecht, The Netherlands, 89–112.
Ticha, I. 1996. Optimization of photoautotrophic tobacco in vitro culture: Effect of suncaps closures on plantlet growth. Photosynthetica 32: 475–479.
Tisserat, B. and Silman, R. 2000. Interactions of culture vessels, media volume, culture density and carbon dioxide levels of lettuce and spearmint shoot growth in vitro. Plant Cell Rep. 19: 464–471.
Van Huylenbroeck, J.M. and Debergh, P.C. 1996. Physiological aspects in acclimatization of micropropagated plantlets. Plant Tiss. Cult. Biotechnol. 2: 136–141.
Vyas, S. and Purohit, S.D. 2003. In vitro growth and shoot multiplication of Wrightia tomentosa Roem et. Schult in a controlled carbon dioxide environment. Plant Cell Tissue Organ Cult. 75: 283–286.
Woltering, E.J. 1986. Ethylene and carbon dioxide accumulation within various tissue culture systems. — Acta. Bot. Neerl. 35: 50.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vyas, S., Purohit, S.D. Effect of controlled carbon dioxide on in vitro shoot multiplication in Feronia limonia (L.) Swingle. Acta Physiol Plant 28, 605–611 (2006). https://doi.org/10.1007/s11738-006-0056-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-006-0056-4