Abstract
The aim of the present studies was to compare H2O2 and ascorbate contents as well as peroxidase (PO) and catalase (CAT) activities in leaves of less susceptible cultivar Perkoz and more susceptible Corindo after B. cinerea infection.
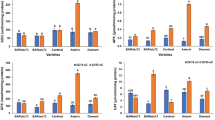
Increase in H2O2 contents in both Perkoz and Corindo cytosol was observed, however, it appeared earlier in the less susceptible cultivar. The increase in PO activity in the cytosol fraction was observed 48 hours after infection in both cultivars but it was greater in the less susceptible Perkoz. No significant differences between the tested cultivars were observed in ascorbate peroxidase (APX) activity and in reduced and oxidated ascorbate contents. PO activity was thoroughly analyzed in the apoplast fraction. It was measured with syringaldazine (S), tetramethylbenzidine (TMB) and ferulic acid (FA)—substrates characteristic of isoenzymes involved in lignification and stiffening of a cell wall. Increase in PO activity with these substrates was observed earlier in cultivar Perkoz than in cultivar Corindo. Similarly, increase in PO activity with NADH appeared significantly earlier in cultivar Perkoz. Apoplastic PO was separated with DEAE Sepharose and two fractions binding and non-binding were obtained. Binding PO fraction was significantly more active especially with S, TMB and NADH after B. cinerea infection. The increase in the enzyme activity was mostly observed in cultivar Perkoz. Binding PO was separated by electrophoresis on acrylamide gel and revealed six enzymatic forms from which three were much more active after infection in cultivar Perkoz. The obtained results suggest that cell wall strengthening mediated by apoplast PO is a key factor responsible for different resistance of tomato cultivars Perkoz and Corindo to B. cinerea infection.
Similar content being viewed by others
References
Baker C.J., Orlandi E.W. 1995. Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33: 299–321.
Baker C.J., O’Neill N.R., Deahl K., Lydon J. 2002. Continuous production of extracellular antioxidants in suspension cells attenuates the oxidative burst detected in plant microbe interactions. Plant Physiol. Biochem. 40: 641–644.
Barcelo R.A., Munoz R., Sabater F. 1987. Lupin peroxidases. I. Isolation nd characterization of cell-wall isoperoxidase activity. Physiologia Plantarum 71: 448–454.
Barcelo R.A. 1998. The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH-oxidase-like enzyme. Planta 207: 207–216.
Blokhina O., Virolainen E., Fageratedt K.V. 2003. Antioxidants, oxidative dmage and oxygen deprivation stress. Rev. Annal Botany 91: 179–194.
Bestwick C.S., Brown I.R., Mansfield J.W. 1998. Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive reaction in lettuce. Plant Physiol. 118: 1067–1078.
Bolwell G.P., Wojtaszek P. 1997. Mechanisms for the generation of reactive oxygen species in plant defence: a broad perspective. Physiol. Mol. Plant Pathol. 51: 347–366.
Bolwell G.P., Blee K.A., Butt V.S., Davies D.R., Gardner S.L., Gerrish D., Minibayeva F., Rowntree E.G., Wojatszek P. 1999. Recent advances in understanding the origin of the apoplastic oxidative burst in plant cells. Free Radic. Res. 31: S137-S145.
Bolwell G.P., Page A., Pi lewska M., Wojtaszek P. 2001. Pathogenic infection and the oxidative defences in plant apoplast. Protoplasma 217: 20–32.
Borden S., Higgins V.J. 2002. Hydrogen peroxide plays a critical role in the defence response of tomato to Cladosporium fulvum. Physiol. Mol. Plant Pathol. 61: 227–236.
Bradford M.M. 1976. A rapid and sensitive msthod for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Capaldi D.J., Taylor K.E. 1983. A new peroxidase colour reaction: oxidative coupling of 3-methyl-2-benzothiazolinone hydrazone (MBTH) with its formaldehyde azine application to glucose and choline oxidases. Anal. Biochem. 129: 329–336.
Chen, S. X., Schopher P. 1999. Hydroxyl-radical production in physiologicalreactions. A novel function of peroxidase. Europ. J. Biochem. 260: 726–735.
Cordoba-Pedregosa M. C., Cordoba F., Villalba J.M., Gonzales-Reyes J.A. 2003. Differential distribution of ascorbic acid, peroxidase activity, and hydrogen peroxide along the root axis Allium cepa L. and its possible relationship with cell growth and differentiation. Protoplasma 221: 57–65.
Creissen G., Firmin J., Freyer M., Kular B., Leyland N., Reynolds H., Pastori G., Wellburn F., Baker N., Wellburn A., Mullineaux P. 1999. Elevated glutathione biosynthetic capacity in the chloroplasts of transgenic tobacca paradoxicaly caused increased oxidative stress. Plant Cell 11: 1277–1291.
Christensen J.H., Bauw G., Welinder K.G., Van Montagu M., Boerjan W. 1998. Purification and characterization of peroxidases correlated with lignification in poplar xylem. Plant Physiol. 118: 125–135.
Dai G.H., Nicole M., Andary C., Martinez C., Bresson E., Boher B., Daniel J.F., Geiger J.P. 1996. Flavonoids accumulate in cell walls, middle lamellae and callose-rich papillae during an incompatible interaction between Xanthomonas campestris pv. Malvacearum and cotton. Physiol. Mol. Plant Pathol. 49: 285–306.
Dhindsa R.S., Plumb-Dhindsa P., Thorpe T.A. 1981. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decrease levels of superoxide dismutase and catalase. J. Exp. Bot. 32: 93–101.
Figueroa-Spinoza M.C., Rouau X. 1998. Oxidative cross-linking of pentosans by a fungal laccase and horse-radish peroxidase: Mechanism of linking between Feruloylated arabinoxylans. Cereal Chem. 75: 259–265.
Frahry G., Schopfer P. 1998. Hydrogen peroxide production by roots and its stimulation by exogenous NADH. Physiol. Plantarum 103: 395–404.
Fry S.C. 1998. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxy radicals. Biochem. J. 332: 507–515.
Genetile I.A., Ferraris L., Matta A. 1988. Variations of phenol oxidase activities as a consequence of stresses that induce resistance to Fusarium wilt of tomato. J. Phytopathol. 122: 45–53.
Goldberg R., Catesson A.M., Czaninski Y. 1983. Some properties of syringaldazine oxidase, a peroxidase specifically involved in the lignification processes. Zeit. F. Pflanzenphysiol. 110: 267–279.
Hammerschmidt R., Nuckles E.M., Ku J. 1982. Asociation of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 20: 73–82.
Ikegawa T., Mayama S., Nakayashiki H., Kato H. 1996. Accumulation of diferulic acid dyring the hypersensitive response of oat leaves to Puccinia coronata f. sp. avenae and its role in the resistance of oat tissues to cell wall degrading enzymes. Physiol. Mol. Plant Pathol. 48: 245–255.
Imberty A., Goldberg R., Catesson A.M. 1985. Isolation and characterization of Populus isoperoxidases involved in the last step of lignification. Planta 164: 221–226.
Ishida A., Ookubu K., Ono K. 1987. Formation of hydrogen peroxide by NAD(P)H oxidation with isolated cell wall-associated peroxidase from cultured liverwort cells, Marchantia polymorpha L. Plant Cell Physiol. 28: 723–726.
Kazan K., Murry F.R., Goulter K.C., Llewellyn D.J., Manners J.M. 1998. Induction of cell death transgenic plants expression a fungal glucose oxidase. Mol. Plant-Microbe Interact. 11: 555–562.
Knörzer O.C., Durner J., Böger P. 1996. Alterations of the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol. Plant. 97: 388–396.
Lamb C., Dixon R.A. 1997. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 251–275.
Liszkay A., Kenk B., Schopfer P. 2003. Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta 217: 658–667.
Maehly A.C., Chance B. 1954. The assay of catalases and peroxidases, [In:] Methods Biochem. Analysis. Glick D. (ed.) 1: 357–425.
Martinez, C., Montilet J.L., Bresson E., Agnel J.P., Dai G.H., Daniel J.F., Geiger J.P., Nicole M. 1998. Apoplastic peroxidase generates superoxide anions in cells of cotton cotyledons undergoing the hypersensitive reaction to Xanthomonas campestris pv. malvacearum Race 18. Mol. Plant-Microbe Ineract. 11: 1038–1047.
Mehlhorn H., Lelandais M., Korth H.G., Foyer C.H. 1996. Ascorbate is natural substrate for plant peroxidases. FEBS Letters 378: 203–206.
Mellersh D.G., Foulds I.V., Higgins J., Heath M.C. 2002. H2O2 plays different roles in determining penetration failure in three diverse plant-fungal interactions. Plant J. 29: 257–268.
Milosevic N., Slusarenko A.J. 1996. Activity oxygen metabolism and lignification in the hypersensitive response in bean. Physiol. Mol. Plant Pathol. 49: 143–158.
Minibayeva F.V., Gordon L.K., Kolesnikov O.P., Chasov A.V. 2001. Role of extracellular peroxidase in the superoxide production by wheat root cells. Protoplasma 217: 125–128.
Nakano Y., Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22: 867–880.
Noctor D., Foyer C.H. 1998. Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 249–279.
O’Connell R.J., Brown I.R., Mansfield J.W., Bailey J.A., Mazau D., Rumeau D., Esquerre-Tugaye M-T. 1990. Immunocytochemical localization of hydroxyproline-rich glycoproteins accumulation in melon and bean at sites of resistance to bacteria and fungi. Mol. Plant Microbe Interact. 3: 33–40.
Ogawa K., Kanematsu S., Asada K. 1997. Generation of superoxide anion and localization of CuZn-superoxide dismutase in the vascular tissue of spinach hypocotyls: their association with lignification. Plant Cell Physiol. 38: 1118–1126.
Olson P.D., Verner J.E. 1993. Hydrogen peroxide and lignification. Plant J. 4: 887–892.
Otter T., Polle A. 1994. The influence of apoplastic ascorbate on the activities of cell wall-associated peroxidase and NADH oxidase in needles of Norway spruce (Picea abies L.). Plant Cell Physiol. 35: 1231–1238.
Pang A., Catesson A.M., Francesch Ch., Rolando Ch., Goldberg R. 1989. On substrate specificity of peroxidases involved in the lignification process. J. Plant Physiol. 135: 325–329.
Patykowski J., Ku niak E. Urbanek H. 1996. Comparison of defence responses to Botrytis cinerea infection in tomato plants propagated in vitro and grown in vivo. Acta Agrobotanica 49: 115–121.
Patykowski J., Urbanek H. 2003. Activity of enzymes related to H2O2 generation and metabolism in leaf apoplastic fraction of tomato cultivars infected with Botrytis cinerea Pers. J. Phytopthol. 151: 153–161.
Peng M., Ku J. 1992. Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf discs. Phytopathplogy 82: 696–699.
Pignocchi C., Foyer Ch.H. 2003. Apoplastic ascorbate metabolism and its role in the regulation of cell signaling. Current Opinion Plant Biology. 6: 379–389.
Sanchez M., Queijeiro E., Revilla G., Zarra I. 1997. Changes in ascorbate acid levels in apoplastic fluid during growth of pine hypocotyls. Effect on peroxidase activities associated with cell walls. Physiol. Plantarum 101: 815–820.
Scott-Craig J.S., Kerby K.B., Stein B.D., Somerville S.C. 1995. Expression of an extracellular peroxidase that is induced in barley (Hordeum vulgare) by the powdery mildew pathogen (Erysiphe graminis f. sp. hordei). Physiol. Mol. Plant Pathol. 47: 407–418.
Schweikert C., Liszkay A., Schopfer P. 2000. Scission of polysaccharide by peroxidase-generated hydroxyl radicals. Phytochemistry 53: 565–570.
Shigeoka S., Ishikawa T., Tamoi M., Miyagawa Y., Takeda T., Yabuta Y., Yoshimura K. 2002. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Botany 53: 1305–1319.
Takahama U. 1995. Oxidation of hydroxycynamic acid and hydroxycynamyl alcohol derivatives by laccase and peroxidase. Interactions among p-hydroxyphenyl, guaiacyl and syringyl groups during the oxidation reactions. Physiol. Plantarum. 93: 61–68.
Thordal-Christensen H., Zhang Z., Wei Y., Collinge D.B. 1997. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 11: 1187–1194.
Van Tiedeman A. 1997. Evidence for a primary role of active oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea. Physiol. Mol. Plant Pathol. 50: 151–166.
Vuletic M., Hadzi-Taskovic V., Jukalovic S. 2000. Characterization of cell wall oxelate from maize roots. Plant Science 157: 257–263.
Wojtaszek P. 1997. Oxidative burst: an early plant response to pathogen infection. Biochem. J. 322: 681–692.
Yurnia T.P., Yurnia E.V., Karavaev V.A., Solntsev M.K., Kukustikina M.A., Ekobena F.A.P. 1996. Physiological characteristics of wheat leaves cultivars resistant and susceptible to powdery mildew. Russ. J. Plant Physiol. 43: 64–69.
Zimmerlin A., Wojtaszek P., Bolwell G.P. 1994. Synthesis of dehydrogenation polymers of ferulic acid with high specificity by a purified cell-wall peroxides from French bean (Phaseolus vulgaris L.). Biochem. J. 299: 747–753.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Patykowski, J., Urbanek, H. The activity of antioxidative enzymes, contents of H2O2 and of ascorbate in tomato leaves of cultivars more or less sensitive to infection with Botrytis cinerea . Acta Physiol Plant 27, 193–203 (2005). https://doi.org/10.1007/s11738-005-0023-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-005-0023-5