Abstract
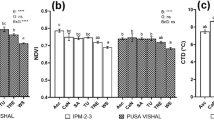
Mung bean and tomato were in vitro selected on media containing 0, 25, 50, 100 and 150 mM NaCl. Two types of media (hormone supplemented media, CB and hormone free media, MS) were used for mung bean using cotyledon explants whereas two types of explants (cotyledons and shoot apices) were used for tomato on MS media. Total-N, protein content, nitrite reductase (NiR) activity and protein protein profiles were checked in selected plants and compared to original non selected ones. NaCl at low concentrations slightly increased total-N in shoots and roots of in vitro selected mung bean and tomato whereas higher concentrations induced significant reductions. Similar increases in protein content were detected at lower concentrations with no significant effects thereover. On the contrary, NaCl gradually inhibited NiR activity. Similar responses of total-N, protein and NiR activity, but with greater magnitudes, were detected in original plants. In addition, NaCl significantly reduced dry weights of shoots and roots of either in vitro selected or, in particular, original intact plants. Moreover, electrophoresis (SDS-PAGE) of protein from shoots of either in vitro selected or intact plants showed that NaCl induced new protein bands while some others were concomitantly disappeared. The induction of one or more of the 86.4, 79, 77.6, 77 and 71.5 kDa bands following in vitro selection and/or the disappearance of the 86 kDa band from intact plants seemed necessary for mung bean resistance. Also, the presence of 86.2 kDa band and/or the loss of the 85.8 and 57.5 kDa bands might be included in tomato resistance. Of these induced bands in mung bean selected on CB media, only two bands were detected in plants selected on MS media. In tomato, two bands lost following selection from cotyledons but only one band lost following selection from shoot apices. These changes in protein pattern therefore might serve as adaptive regulators for resistance to NaCl.
Similar content being viewed by others
References
Abd El-Samad HM 2002. Salt tolerance and interaction with abscisic acid of tomato cultivars. Bull. Fac. Sci. Assiut Univ. 31: 205–217.
Abdel-Hady MS 2001. Wheat plantlets production via shoot tips under salinity stress. J. Agric. Sci. 26: 4841–4857.
Albassam BA 2001. Effect of nitrate nutrition on growth and nitrogen assimilation of pearl millet exposed to sodium chloride stress. J. Plant Nutr. 24: 1325–1335.
Ashraf M, Rasul E 1988. Salt tolerance of mung bean (Vigna radiata (L.) Wilczek) at two growth stages. Plant and Soil 110: 63–67.
Bourgeais CP, Guerrier G 1992. Salt-responses in Lycopersicon esculentum calli and whole plants. J. Plant Physiol. 140: 494–501.
Bradford MM 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–154.
Bray EA 1991. Regulation of gene expression by endogenous ABA during drought stress. In: Abscisic Acid Physiology and Biochemistry. W.J. Davis, H.G. Jones (Eds.) Bio. Scientific Publishers (Pubs). Lancaster, U.K. p81.
Cano EA, Perez-Alfocea F, Moreno V, Caro M, Bolarin MC 1998. Evaluation of salt tolerance in cultivated wild tomato species through in vitro shoot apex culture. Plant Cell, Tissue and Organ Cult. 53: 19–26.
Chen YZ, Wang YG 1992. The reaction of soyabean callus tissue and regenerated plantlets to salt stress. Soybean Sci. 11: 70–73.
Cusido RM, Palazon J, Altabella T, Morales C 1987. Effect of salinity on soluble protein, free amino acids and nicotine contents in Nicotiana rustica L. Plant and Soil 102: 55–60.
Dell’Aquila A, Spada P 1993. The effect of salinity stress upon protein synthesis of germinating wheat embryos. Ann. Bot. 72: 97.
Delory M 1949. Colourimetric estimation of ammonia. Vogel Inorganic Chemistry. Longman group Ltd., London.
El-Bagoury OH, El-Agroudy MH, Shenouda MA 1993. Effect of salinity levels on chemical composition of some plant species. Egypt. J. Agron. 18: 95–111.
El-Enany AE 1997. Glutathione metabolism in soybean callus-culture as affected by salinity. Biol. Plant. 39: 35–39.
Garcia AB, Engler JA, Lyer S, Gerats T, Mantagu MV, Caplan AB 1997. Effect of osmoprotectants upon NaCl stress in rice. Plant. Physiol. 115: 159.
Garg BK, Garg OP 1980. Sodium carbonate and bicarbonate induced changes in growth, chlorophyll, nucleic acids and protein contents in leaves of Pisum sativum. Photosynthetica 14: 594.
Gulati A, Jaiwal PK 1990. Culture conditions effecting plant regeneration from cotyledon explants of Vigna radiata (L.) Wilczek. Plant Cell, Tissue and Organ Cult. 23: 1–7.
Gulati A, Jaiwal PK, 1993. In vitro selection of salt-resistant Vigna radiata (L.) Wilczek plants by adventitious shoot formation from cultured cotyledon explants. J. Plant Physiol. 142: 99–102.
Gupta SD 1999. Protein profiles of somatic embryos and regenerated plants from NaCl selected and control cultures of orchardgrass. Biol. Plant. 42: 297–302.
Harborne JB 1988. Phytochemical Methods. Chapman and Hall, London, pp 243.
Hurkman WJ, Tao HP, Tanaka CK 1991. Germinlike polypeptides increase in barley roots during salt stress. Plant Physiol. 97: 366–374.
Her SE, Swanton CJ, Peter Pauls K 1993. In vitro selection of Imazthapyr- tolerant tomato (Lycopersicon esculentum mill). Weed Sci. 41: 12–17.
Imamul-Huq SM, Larher F 1983. Osmoregulation in higher plants: Effect of NaCl salinity on non-nodulated Phaseolus aureus L. I. Growth and mineral content. New Phytol. 93: 203–208.
Khan MG 1996. Nitrate and nitrite reductase activities in soybean plants raised with saline water. Indian J. Plant Physiol. 1: 128–129.
Laemmli UK 1970. Cleavage of structural proteins during the assembly of the heat of bacteriophage T4. Nature 227: 680–685.
Munns R, Termaat A 1986. Whole-plant responses to salinity. Aust. J. Plant Physiol. 13: 143–160.
Munoz GE, Marin K, Gonzalez C 1997. Polypeptide profile in Prosopis seedlings growing in saline conditions. Phyton 61: 17–24.
Murashige T, Skoog F 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 472–497.
Nagaoka S, Hirasawa M, Fukushima K, Tamura G 1984. Methyle viologen linked nitrite reductase from bean roots. Agric. Biol. Chem. 48: 1179–1188.
Nesiem MR, Ghallab AM 1998. Interactive effects of ABA and salinity on growth and yield of two wheat cultivars (Triticum aestivum). Proceedings, Sixth Egyptian Botanical Conference Cairo Univ. 1: 133–154.
Nesiem MR, Ghallab AM 1999. Interactive effects of ABA and salinity on growth and yield of two wheat cultivars. J. Agric. Sci. 24: 625–651.
Perez-Alfocea F, Estan MT, Caro M, Guerrier G 1993. Osmotic adjustment in Lycopersicon esculentum and L. pennellii under NaCl and polyethylene glycol 6000 iso-osmotic stresses. Physiol. Plant. 87: 493–498.
Pollard A, Wyn-Jones RG 1979. Enzyme activities in concentrated solutions of glycinebetaine and other solutes. Planta 144: 291.
Snedecor W, Cochran G 1980. Statistical methods. 7th ed. The Iowa State Univ. Press, USA.
Sumaryati SI, Negrutiu I, Jacobs M 1992. Characterization and regeneration of salt- and water-stress mutants from protoplast culture of Nicotana plumbaginifolia (Vivani). Thear. Appl. Genet. 83: 613–619.
Termaat A, Munns R 1986. Use of concentrated macroelements solution to separate osmotic from NaCl specific effects on plant growth. Aust. J. Plant Physiol. 13: 509–522.
Wray JL, Filner P 1970. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem. J. 119: 715–725.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassan, N.M., Serag, M.S. & El-Feky, F.M. Changes in nitrogen content and protein profiles following in vitro selection of NaCl resistant mung bean and tomato. Acta Physiol Plant 26, 165–175 (2004). https://doi.org/10.1007/s11738-004-0006-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-004-0006-y