Abstract
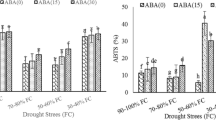
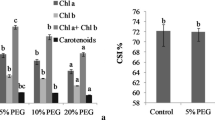
The effect of abscisic acid (ABA) treatment on growth pigments and antioxidant defense system were investigated in seedlings of Helianthus annuus (cvs. Nantio F1 and Özdemirbey) subjected to drought and waterlogging stress. In addition, seedlings were sprayed with 10 M ABA three times every other day. Relative growth rate (RGR) was significantly reduced in both genotypes under drought stress, however, this growth inhibition was less in ABA-treated plants. Total chlorophyll content increased by drought stress in both genotypes. Ascorbate was not influenced by drought, while α-tocopherol increased in cv. Nantio F1. Ascorbate and α-tocopherol increased with drought stress in cv. Özdemirbey. ABA treatment decreased ascorbate and β-carotene contents while it increased α-tocopherol and xanthophylls contents under drought stress. The activity of superoxide dismutase (SOD) in both genotypes increased under drought stress-ABA combinations. Catalase (CAT) activity decreased under drought stress and drought-ABA combinations while it increased under waterlogging stress. Glutathione reductase (GR) activity decreased under drought stress but recovered with ABA treatment. The results suggested that ABA treatments have different effects on the components of antioxidant defense system in H. annuus genotypes and ABA may contribute drought-induced oxidative stress tolerance but not effects under waterlogging stress.
Similar content being viewed by others
References
Aebi H.E. 1983. Catalase. In: methods of enzymatic analysis. Bergmeyer, J., Grabi, M., Eds. Verlag Chemie. Weinheim., 3: 273–286.
Ahmed S., Nawata E., Hosokawa Y., Domae T., Sakuratani T. 2002. Alterations in photosynthesis and antioxidant enzymatic activities of mungbean subjected to waterlogging. Plant Sci., 163: 117–123.
Alscher R.G., Erturk N., Heath L.S. 2002. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot., 53: 1331–1341.
Asada K. 1996. Radical production and scavenging in chloroplasts. In: Baker NR (ed) Photosynthesis and Environment. Kluwer Academic Publisher, Dordrecht, The Netherlands. 123–150.
Bacon M.A. 1999. The biochemical control of leaf expansion during drought. Plant Growth Regul., 29: 101–112.
Bailly C., Audigier C., Ladonne F., Wagner M.H., Coste F., Corbineau F., Côme D. 2001. Changes in oligosaccharide content and antioxidant enzyme activities in developing bean seeds as related to acquisition of drying tolerance and seed quality. J. Exp. Bot., 52: 701–708.
Behera R.K., Mishra P.C., Choudhury N.K. 2002. High irradiance and water stress induce alterations in pigment composition and chloroplast activities of primary wheat leaves. J. Plant Physiol., 159: 967–973.
Beyer W.F., Fridowich I. 1987. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem., 161: 559–566.
Bonnet M., Camares O., Veisseire P. 2000. Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L.cv Apollo). J. Exp. Bot., 51: 945–953.
Bowler C., Van Montagu M., Inze D. 1992. Superoxide dismutase and stress tolerance. Annu. Rev. Plant. Physiol. Plant. Mol. Biol., 43: 83–116.
Caretto S., Paradiso A., D’Amico L., De Gara L. 2002. Ascorbate and glutathione metabolism in two sunflower cell lines of differing α-tocopherol biosynthetic capability. Plant Physiol. Biochem., 40: 509–513.
Carlberg I., Mannervik B. 1985. Glutathione reductase. Methods Enzymol., 113: 484–490.
Chaitanya K.V., Sundar D., Masilamani S., Ramachandra Reddy A. 2002. Variation in heat stressinduced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul., 36: 175–180.
Herbinger K., Tausz M., Wonisch A., Soja G., Sorger A., Grill D. 2002. Complex interactive effects of drought and ozone stress on the antioxidant defense systems of two wheat cultivars. Plant Physiol. Biochem., 40: 691–696.
Jiang M., Zhang J. 2001. Effect of abscisic acid on active oxygen species, antioxidative defense system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol., 42: 1265–1273.
Jiang M., Zhang J. 2002. Involvement of plasmamembrane NADPH oxidase in abscisic acid-and water stress-induced antioxidant defense in leaves of maize seedlings. Planta, 215: 1022–1030.
Kele Y., Öncel I. 2000. Changes of superoxide dismutase activity in wheat seedlings exposed to natural environmental stresses. Commun Fac. Sci. Univ. Ank. Ser. C., 18: 1–8.
Kele Y., Öncel I. 2002. Response of antioxidative defense system to temperature and water stress combinations in wheat seedlings. Plant Sci., 163: 783–790.
Moore T.C. 1974. Research experiences in plant physiology. Springer-Verlag, New-York.
Moran J.F., Bacana M., Iturbe-Ormaetxe I., Frechilla S., Klucas R.V., Aparicio-Jejo P. 1994. Drought induces oxidative stress in pea plants. Planta, 194: 346–352.
Muller, H.H., Marschner H. 1997. Use of an in vitro assay to investigate the antioxidative defense potential of wheat genotypes under drought stress as influenced by nitrogen nutrition. Phyton (Austria)., 37: 187–196.
Pastori G.M., Foyer C.H. 2002. Common components, networks, and pathways of cross-tolerance to stress. The central role of “redox” and abscisic acid-mediated controls. Plant Physiol., 129: 460–468.
Porra R.J., Thompson R.A., Kriedemann P.E. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochem. Biophys. Acta., 975: 384–394.
Rubio M.C., González E.M., Minchin F.R., Webb K.J., Arrese-Igor C., Ramos J., Becana M. 2002. Effects of water stress on antioxidant enzymes of leaves and nodules of transgenic alfalfa overexpressing superoxide dismutases. Physiol. Plant., 115: 531–540.
Sánchez-Blanco M.J., Rodríguez M.A., Morales M.A., Ortu aM.F., Torrecillas A. 2002. Comparative growth and water relations of Cistus albidus and Cistus monspeliensis plants during water deficit conditions and recovery. Plant Sci., 162: 107–113.
Schmieden U., Wild A. 1994. Changes in levels of α-tocopherol and ascorbate in spruce needles at three low mountain sites exposed to Mg+2 deficiency and ozone. Z. Naturforsch., 49: 171–180.
Schurr U., Heckenberger U., Herdel K., Walter A., Feil R. 2000. Leaf development in Ricinus communis during drought stress: dynamics of growth processes, of cellular structure and of sink-source transition. J. Exp. Bot., 51: 1515–1529.
Snowden R.E.D., Wheeler B.D. 1993. Iron toxicity to fen plant species. J. Ecology, 81: 35–46.
Synková H., Pospíšilová J. 2002. In vitro precultivation of tobacco affects the response of antioxidative enzymes to ex vitro acclimation. J. Plant Physiol., 159: 781–789.
Van Der Mescht A., De Ronde J.A., Ross F.T. 1998. Cu/Zn superoxide dismutase, glutathione reductase and ascorbate peroxidase levels during drought stress in potato. S. African J. Sci., 94: 496–500.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kele, Y., Ünyayar, S. Responses of antioxidant defense system of Helianthus annuus to abscisic acid treatment under drought and waterlogging. Acta Physiol Plant 26, 149–156 (2004). https://doi.org/10.1007/s11738-004-0004-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-004-0004-0