Abstract
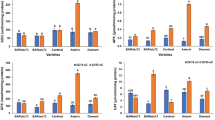
Seedlings of lupine (Lupinus luteus L. cv. Juno) were exposed for up to 96 hours to 1 to 2 kPa partial pressure oxygen (hypoxic treatment) and activities of alcohol dehydrogenase (ADH), lactate dehydrogenase (LDH) and their isoform profiles were determined. Roots of lupine seedlings were grown in a nitrogen flushed nutrient solution while their shoots were in air. Prolonged hypoxia led to a reduction of root elongation. This was accompanied by reduced increase in dry weight suggesting that insufficient carbohydrate supply was the cause of retarded growth of lupine roots. Hypoxically treated roots showed induction of ADH and LDH acivities. The maximum increase in LDH activity was low (2-fold) in contrast to ADH activity, which increased up to 7-fold. Hypoxic treatment of roots did not affect the activities of ADH and LDH in hypocotyls and cotyledons. Analysis of ADH and LDH activity gels indicated in roots 1 and 2 isoforms, respectively. The level of isozymes of both enzymes increased in roots upon exposure to hypoxic stress. Differences in isoenzymatic spectrum of ADH and LDH between roots, hypocotyls and cotyledons indicate organ specificity of isozymes of both enzymes. The importance of alcohol and lactate fermentation in roots to cope with hypoxic stress is discussed.
Similar content being viewed by others
Abbreviations
- ADH:
-
alcohol dehydrogenase
- LDH:
-
lactate dehydrogenase
References
Armstrong W. 1979. Aeration in higher plants. Adv. Bot. Res. 7: 225–232
Bacanamwo M., Purcell L.C. 1999. Soybean root morphological and anatomical traits associated with acclimation to flooding. Crop Sci. 39: 143–149
Bradford M. 1976. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72: 248–254.
Chourey P., Widholm J. 1980. Tissue specific alcohol dehydrogenase isozyme variation in carrot: whole plant versus in vitro cultured cells. In vitro 16: 571–574
Christopher M.E., Good A.G. 1996. Characterization of hypoxically inducible lactate dehydrogenase in maize. Plant Physiol. 112:1015–1022
Czosnowski J. 1974. Metabolism of excised embryos of Lupinus luteus L. An electrophoretic analysis of some dehydrogenases in cultured embryos as compared with the normal seedling axes. Acta Soc. Bot. Pol. 43: 117–127
Davies D.D., Grego S., Kenworthy P. 1974. The control of the production of lactate and ethanol by higher plants. Planta 118: 297–310
Drew M.C. 1997. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48:223–250
Garnczarska M., Ratajczak L. 1993. Changes in the activity and isozyme patterns of alcohol dehydrogenase in developing and germinating seeds of yellow lupine. Acta Physiol. Plant. 15: 257–261
Good A.G., Crosby W.L. 1989. Induction of alcohol dehydrogenase and lactate dehydrogenase in hypoxically induced barley. Plant Physiol. 90: 860–866
Good A.G., Muench D.G. 1993. Long-term anaerobic metabolism in root tissue. Metabolic products of pyruvate metabolism. Plant Physiol. 101: 1163–1168
Gottlieb L.P. 1982. Conservation and duplication of isozymes in plants. Science 216: 373–380
Hanson A.D., Jacobsen J.V., Zwar J.A. 1984. Regulated expression of three alcohol dehydrogenase genes in barley aleurone layers. Plant Physiol. 75: 573–581
Harberd N.P., Edwards K.J.R. 1982. The effect of the mutation causing alcohol dehydrogenase deficiency on flooding tolerance in barley. New Phytol. 90: 631–644
Hoffman N.E., Bent A.F., Hanson A.D. 1986. Induction of lactate dehydrogenase isozymes by oxygen deficit in barley root tissue. Plant Physiol. 82: 658–663
Jackson M.B., Davies D.D., Lambers H. 1991. Plant Life under Oxygen Deprivation: Ecology, Physiology and Biochemistry. SPB Academic, The Hague, The Netherlands: 72–90
Jackson M.B., Herman B., Goodenough A. 1982. An examination of the importance of ethanol in causing injury to flooded plants. Plant Cell Environ. 8: 163–172
Kennedy R.A., Rumpho M.E., Fox T.C. 1992. Anaerobic metabolism in plants. Plant Physiol. 100: 1–6
Kundu C., Banerji C., Banerji B., Mandal B.K., Mallik S. 1993. Amount of volatile aldehydes released by rice plants after submergence. IRRN 18: 19–20
Laemmli U.K. 1970. Cleavage of structual proteins during assembly of the head of bacteriophage T4. Nature 227: 680–685
Matsumura H., Takano T., Yoshida K.T., Takeda G. 1995. A rice mutant lacking alcohol dehydrogenase. Breed Sci. 45: 365–367
Minhas D., Grover A. 1999. Transcript levels of gene encoding various glycolytic and fermentation enzymes change in response to abiotic stresses. plant Sci. 146: 41–51
Perata P., Alpi A. 1993. Plant responses to anaerobiosis. Plant Sci. 93: 1–17
Ricard B., Couee I., Raymond P., Saglio P.H., Saint-Ges V., Pradet A. 1994. Plant metabolism under hypoxia and anoxia. Plant Physiol. Bioch. 32: 1–10
Rivoal J., Hanson A.D. 1994. Metabolic control of anaerobic glycolysis. Overexpression of lactate dehydrogenase in transgenic tomato roots supports the Davies-Roberts hypothesis and points to a critical role for lactate secretion. Plant Physiol. 106: 1179–1185
Sachs M.M., Freeling M., Okimoto R. 1980. The anaerobic proteins of maize. Cell 20: 761–767
Sachs M.M., Ho T.H.D. 1986. Alteration of gene expression during environmental stress in plants. Annu. Rev. Plant Physiol. 37: 363–376
Sachs M.M., Subbaiah C.C., Saab I.N. 1996. Anaerobic gene expression and flooding tolerance in maize. J Exp. Bot. 47: 1–15
Sakano K. 2001. Metabolic regulation of pH in plant cells: Role of cytoplasmic pH in defense reaction and secondary metabolism. Inter. Rev. Cyt. 206: 1–44
Setter T.L., Laureles E.V. 1996. The beneficial effect of reduced elongation growth on submergence tolerance of rice. J Exp. Bot. 47: 1551–1559
VanToai T.T., Saglio P., Ricard B., Pradet A. 1995. Developmental regulation of anoxic stress tolerance in maize. Plant Cell Environ. 18: 937–942
Vartapetian B.B., Jackson M.B. 1997. Plant adaptation to anaerobic stress. Ann. Bot. 79: 3–20
Waters I., Morrell S., Greenway H., Colmer T.D. 1991. Effects of anoxia on wheat seedlings. II. Influence of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation, and sugar levels. J.Exp. Bot. 42: 1437–1447
Xia J.H., Saglio P.H. 1992. Lactic acid efflux as a mechanism of hypoxic acclimation of maize root tips to anoxia. Plant. Physiol. 100: 40–46
Xie Y., Wu R. 1989. Rice alcohol dehydrogenase genes: anaerobic induction, organ specific expression and characterization of cDNA clones. Plant Mol. Biol. 13: 53–68
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garnczarska, M. Hypoxic induction of alcohol and lactate dehydrogenases in lupine seedlings. Acta Physiol Plant 24, 265–272 (2002). https://doi.org/10.1007/s11738-002-0050-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-002-0050-4