Abstract
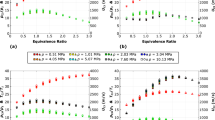
The shock tube autoignition of 2,5-dimethylfuran (DMF)/n-heptane blends (DMF0-100%, by mole fraction) with equivalence ratios of 0.5, 1.0, and 2.0 over the temperature range of 1200–1800 K and pressures of 2.0 atm and 10.0 atm were investigated. A detailed blend chemical kinetic model resulting from the merging of validated kinetic models for the components of the fuel blends was developed. The experimental observations indicate that the ignition delay times nonlinearly increase with an increase in the DMF addition level. Chemical kinetic analysis including radical pool analysis and flux analysis were conducted to explain the DMF addition effects. The kinetic analysis shows that at lower DMF blending levels, the two fuels have negligible impacts on the consumption pathways of each other. As the DMF addition increases to relatively higher levels, the consumption path of n-heptane is significantly changed due to the competition of small radicals, which primarily leads to the nonlinear increase in the ignition delay times of DMF/nheptane blends.
Similar content being viewed by others
References
Zhong S, Daniel R, Xu H, Zhang J, Turner D, Wyszynski M L, Richards P. Combustion and emissions of 2,5-dimethylfuran in a direct-injection spark-ignition engine. Energy & Fuels, 2010, 24(5): 2891–2899
Somers K P, Simmie J M, Gillespie F, Conroy C, Black G, Metcalfe W K, Battin-Leclerc F, Dirrenberger P, Herbinet O, Glaude P A, Dagaut P, Togbé C, Yasunaga K, Fernandes R X, Lee C, Tripathi R, Curran H J. A comprehensive experimental and detailed chemical kinetic modelling study of 2,5-dimethylfuran pyrolysis and oxidation. Combustion and Flame, 2013, 160(11): 2291–2318
Sirjean B, Fournet R, Glaude P A, Battin-Leclerc F, Wang W, Oehlschlaeger M A. Shock tube and chemical kinetic modeling study of the oxidation of 2,5-dimethylfuran. Journal of Physical Chemistry A, 2013, 117(7): 1371–1392
Román-Leshkov Y, Barrett C J, Liu Z Y, Dumesic J A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature, 2007, 447(7147): 982–985
Zhao H, Holladay J E, Brown H, Zhang Z C. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural. Science, 2007, 316(5831): 1597–1600
Daniel R, Tian G, Xu H, Wyszynski M L, Wu X, Huang Z. Effect of spark timing and load on a DISI engine fuelled with 2,5-dimethylfuran. Fuel, 2011, 90(2): 449–458
Daniel R, Wei L, Xu H, Wang C, Wyszynski M L, Shuai S. Speciation of hydrocarbon and carbonyl emissions of 2,5-dimethylfuran combustion in a DISI engine. Energy & Fuels, 2012, 26(11): 6661–6668
Rothamer D A, Jennings J H. Study of the knocking propensity of 2,5-dimethylfuran-gasoline and ethanol-gasoline blends. Fuel, 2012, 98: 203–212
Gouli S, Lois E, Stournas S. Effects of some oxygenated substitutes on gasoline properties, spark ignition engine performance, and emissions. Energy & Fuels, 1998, 12(5): 918–924
Zhang Q, Chen G, Zheng Z, Liu H, Xu J, Yao M. Combustion and emissions of 2,5-dimethylfuran addition on a diesel engine with low temperature combustion. Fuel, 2013, 103: 730–735
Li J, Yang W M, An H, Zhou D Z, Yu W B, Wang J X, Li L. Numerical investigation on the effect of reactivity gradient in an RCCI engine fueled with gasoline and diesel. Energy Conversion and Management, 2015, 92: 342–352
Benajes J, Molina S, García A, Monsalve-Serrano J. Effects of low reactivity fuel characteristics and blending ratio on low load RCCI (reactivity controlled compression ignition) performance and emissions in a heavy-duty diesel engine. Energy, 2015, 90: 1261–1271
Li J, Yang W, Zhou D. Review on the management of RCCI engines. Renewable & Sustainable Energy Reviews, 2017, 69: 65–79
Ma S, Zheng Z, Liu H, Zhang Q, Yao M. Experimental investigation of the effects of diesel injection strategy on gasoline/diesel dual-fuel combustion. Applied Energy, 2013, 109: 202–212
Walker N R, Dempsey A B, Andrie M J, Reitz R D. Experimental study of low-pressure fueling under RCCI engine operation. In: Paper ILASS2012–80, ILASS-Americas 24th Annual Conference on Liquid Atomization and Spray Systems, 2012
Hanson R, Reitz R D. Transient RCCI operation in a light-duty multi-cylinder engine. SAE International Journal of Engines, 2013, 6(3): 1694–1705
Curran S, Prikhodko V, Cho K, Sluder C S, Parks J, Wagner R, Kokjohn S, Reitz R D. In-cylinder fuel blending of gasoline/diesel for improved efficiency and lowest possible emissions on a multicylinder light-duty diesel engine. SAE Technical Paper, 2010
Wu X, Huang Z, Jin C, Wang X, Zheng B, Zhang Y, Wei L. Measurements of laminar burning velocities and markstein lengths of 2,5-dimethylfuran-air-diluent premixed flames. Energy & Fuels, 2009, 23(9): 4355–4362
Wu X, Huang Z, Jin C, Wang X, Wei L. Laminar burning velocities and markstein lengths of 2,5-dimethylfuran-air premixed flames at elevated temperatures. Combustion Science and Technology, 2010, 183(3): 220–237
Wu X, Huang Z, Wang X, Jin C, Tang C, Wei L, Law C K. Laminar burning velocities and flame instabilities of 2,5-dimethylfuran–air mixtures at elevated pressures. Combustion and Flame, 2011, 158 (3): 539–546
Simmie J M, MetcalfeWK. Ab initio study of the decomposition of 2,5-dimethylfuran. Journal of Physical Chemistry A, 2011, 115(32): 8877–8888
Sirjean B, Fournet R. Unimolecular decomposition of 2,5-dimethylfuran: a theoretical chemical kinetic study. Physical Chemistry Chemical Physics, 2013, 15(2): 596–611
Simmie J M, Curran H J. Formation enthalpies and bond dissociation energies of Alkylfurans. The strongest CsX bonds known? Journal of Physical Chemistry, 2009, 113(17): 5128–5137
Qian Y, Zhu L, Wang Y, Lu X. Recent progress in the development of biofuel 2,5-dimethylfuran. Renewable & Sustainable Energy Reviews, 2015, 41: 633–646
Vermeer D J, Meyer J W, Oppenheim A K. Auto-ignition of hydrocarbons behind reflected shock waves. Combustion and Flame, 1972, 18(3): 327–336
Coats C M, Williams A. Investigation of the ignition and bomcustion of n-heptane-oxygen mixtures. Proceedings of the Combustion Institute, 1979, 17(1): 611–621
Ciezki H K, Adomeit G. Shock-tube investigation of self-ignition of n-heptane-air mixtures under engine relevant conditions. Combustion and Flame, 1993, 93(4): 421–433
Herzler J, Jerig L, Roth P. Shock tube study of the ignition of lean nheptane/ air mixtures at intermediate temperatures and high pressures. Proceedings of the Combustion Institute, 2005, 30(1): 1147–1153
Davidson D F, Hong Z, Pilla G L, Farooq A, Cook R D, Hanson R K. Multi-species time-history measurements during n-dodecane oxidation behind reflected shock waves. Proceedings of the Combustion Institute, 2011, 33(1): 151–157
Zhang J, Niu S, Zhang Y, Tang C, Jiang X, Hu E, Huang Z. Experimental and modeling study of the auto-ignition of n-heptane/ n-butanol mixtures. Combustion and Flame, 2013, 160(1): 31–39
Westbrook C K, Warnatz J, Pitz W J. A detailed chemical kinetic reaction mechanism for the oxidation of iso-octane and n-heptane over an extended temperature range and its application to analysis of engine knock. Proceedings of the Combustion Institute, 1989, 22(1): 893–901
Curran H J, Gaffuri P, Pitz W J, Westbrook C K. A Comprehensive modeling study of n-heptane oxidation. Combustion and Flame, 1998, 114(1–2): 149–177
Mehl M, PitzW J, Westbrook C K, Curran H J. Kinetic modeling of gasoline surrogate components and mixtures under engine conditions. Proceedings of the Combustion Institute, 2011, 33(1): 193–200
Herbinet O, Husson B, Serinyel Z, Cord M, Warth V, Fournet R, Glaude P A, Sirjean B, Battin-Leclerc F, Wang Z, Xie M, Cheng Z, Qi F. Experimental and modeling investigation of the lowtemperature oxidation of n-heptane. Combustion and Flame, 2012, 159(12): 3455–3471
Pelucchi M, Bissoli M, Cavallotti C, Cuoci A, Faravelli T, Frassoldati A, Ranzi E, Stagni A. Improved Kinetic model of the low-temperature oxidation of n-heptane. Energy & Fuels, 2014, 28 (11): 7178–7193
Zhang K, Banyon C, Bugler J, Curran H J, Rodriguez A, Herbinet O, Battin-Leclerc F, B’Chir C, Heufer K A. An updated experimental and kinetic modeling study of n-heptane oxidation. Combustion and Flame, 2016, 172: 116–135
Horning D C, Davidson D F, Hanson R K. Study of the hightemperature autoignition of n-alkane/O/Ar mixtures. Journal of Propulsion and Power, 2002, 18(2): 363–371
Herzler J, Naumann C. Shock-tube study of the ignition of methane/ ethane/hydrogen mixtures with hydrogen contents from 0%to 100% at different pressures. Proceedings of the Combustion Institute, 2009, 32(1): 213–220
Lutz A E, Kee R J, Miller J A. SENKIN: a Fortran program for predicting homogeneous gas phase chemical Kinetics with sensitivity analysis.1988-02-01, https://www.osti.gov/biblio/5371815-senkin-fortran-program-predicting-homogeneous-gas-phase-chemical-kinetics-sensitivity-analysis
Kee R J, Rupley F M, Miller J A. CHEMKIN-II: a Fortran chemical kinetics package for the analysis of gas-phase chemical kinetics. 1989-09-01, https://www.osti.gov/biblio/5681118-chemkin-ii-fortran-chemical-kinetics-package-analysis-gas-phase-chemicalkinetics
Xu N, Tang C, Meng X, Fan X, Tian Z, Huang Z. Experimental and kinetic study on the ignition delay times of 2,5-dimethylfuran and the comparison to 2-methylfuran and furan. Energy & Fuels, 2015, 29(8): 5372–5381
Hu E, Chen Y, Zhang Z, Pan L, Li Q, Cheng Y, Huang Z. Experimental and kinetic study on ignition delay times of dimethyl carbonate at high temperature. Fuel, 2015, 140: 626–632
Chen Z, Qin X, Ju Y, Zhao Z, Chaos M, Dryer F L. High temperature ignition and combustion enhancement by dimethyl ether addition to methane–air mixtures. Proceedings of the Combustion Institute, 2007, 31(1): 1215–1222
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos. 91641124, 51306144) and the Project of Youth Star in Science and Technology of Shaanxi Province (2018KJXX-031). The supports from the Fundamental Research Funds for the Central Universities and the State Key Laboratory of Engines at Tianjin University (K2018-10) are also appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, Z., Hu, E., Xu, Z. et al. Effect of 2,5-dimethylfuran addition on ignition delay times of n-heptane at high temperatures. Front. Energy 13, 464–473 (2019). https://doi.org/10.1007/s11708-019-0609-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11708-019-0609-z