Abstract
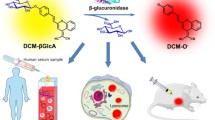
Uridine diphosphate (UDP)-glucuronosyl-transferases (UGTs) are enzymes involved in the biotransformation of important endogenous compounds such as steroids, bile acids, and hormones as well as exogenous substances including drugs, environmental toxicants, and carcinogens. Here, a novel fluorescent probe BDMP was developed based on boron-dipyrromethene (BODIPY) with high sensitivity for the detection of UGT1A8. The glucuronidation of BDMP not only exhibited a red-emission wavelength (λex/λem = 500/580 nm), but also displayed an excellent UGT1A8-dependent fluorescence signal with a good linear relationship with UGT1A8 concentration. Based on this perfect biocompatibility and cell permeability, BDMP was successfully used to image endogenous UGT1A8 in human cancer cell lines (LoVo and HCT15) in real time. In addition, BDMP could also be used to visualize UGT1A8 in tumor tissues. These results suggested that BDMP is a promising molecular tool for the investigation of UGT1A8-mediated physiological function in humans.

Similar content being viewed by others
References
Tian X G, Liang S C, Wang C, Wu B J, Ge G B, Deng S, Liu K X, Yang L, Ma X C. Regioselective glucuronidation of andrographolide and its major derivatives: metabolite identification, isozyme contribution, and species differences. AAPS Journal, 2015, 17(1): 156–166
Kiang T K, Ensom M H, Chang T K. UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacology & Therapeutics, 2005, 106(1): 97132
Oda S, Fukami T, Yokoi T, Nakajima M. A comprehensive review of UDP-glucuronosyltransferase and esterases for drug development. Drug Metabolism and Pharmacokinetics, 2015, 30(1): 30–51
Knights K M, Miners J O. Renal UDP-glucuronosyltransferases and the glucuronidation of xenobiotics and endogenous mediators. Drug Metabolism Reviews, 2010, 42(1): 63–73
Mu J, He L, Huang P, Chen X Y. Engineering of nanoscale coordination polymers with biomolecules for advanced applications. Coordination Chemistry Reviews, 2019, 399: 213039
Izukawa T, Nakajima M, Fujiwara R, Yamanaka H, Fukami T, Takamiya M, Aoki Y, Ikushiro S, Sakaki T, Yokoi T. Quantitative analysis of UDP-glucuronosyltransferase (UGT) 1A and UGT2B expression levels in human livers. Drug Metabolism and Disposition: the Biological Fate of Chemicals, 2009, 37(8): 1759–1768
Nakamura A, Nakajima M, Yamanaka H, Fujiwara R, Yokoi T. Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metabolism and Disposition: the Biological Fate of Chemicals, 2008, 36(8): 1461–1464
Wu B J, Kulkarni K, Basu S, Zhang S X, Hu M. First-pass metabolism via UDP-glucuronosyltransferase: a barrier to oral bioavailability of phenolics. Journal of Pharmaceutical Sciences, 2011, 100(9): 3655–3681
Mackenzie P I, Bock K W, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners J O, Owens I S, Nebert D W. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenetics and Genomics, 2005, 15(10): 677–685
Sanchez-Dominguez C N, Gallardo-Blanco H L, Salinas-Santander M A, Ortiz-Lopez R. Uridine 5′-diphospho-glucronosyltrasferase: its role in pharmacogenomics and human disease. Experimental and Therapeutic Medicine, 2018, 16(1): 3–11
Zhang R Y, Cui Y L, Wang Y, Tian X G, Zheng L, Cong H J, Wu B, Huo X K, Wang C, Zhang B J, Wang X, Yu Z. Catechol-O-methyltransferase and UDP-glucuronosyltransferases in the metabolism of baicalein in different species. European Journal of Drug Metabolism and Pharmacokinetics, 2017, 42(6): 981–992
Xu M, Dong P P, Tian X G, Wang C, Huo X K, Zhang B J, Wu L J, Deng S, Ma X C. Drug interaction study of natural steroids from herbs specifically toward human UDP-glucuronosyltransferase (UGT) 1A4 and their quantitative structure activity relationship (QSAR) analysis for prediction. Pharmacological Research, 2016, 110: 139–150
de Boer Y S, Sherker A H. Herbal and dietary supplement—induced liver injury. Clinics in Liver Disease, 2017, 21(1): 135–149
Tukey R H, Strassburg C P. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annual Review of Pharmacology and Toxicology, 2000, 40(1): 581–616
Tukey R H, Strassburg C P. Genetic multiplicity of the human UDP-glucuronosyltransferases and regulation in the gastrointestinal tract. Molecular Pharmacology, 2001, 59(3): 405–414
Lehmann L, Wagner J. Gene expression of 17β-estradiol-metabolizing isozymes: comparison of normal human mammary gland to normal human liver and to cultured human breast adenocarcinoma cells. Advances in Experimental Medicine and Biology, 2008, 617: 617–624
Zhao F, Wang X, Wang Y, Zhang J B, Lai R, Zhang B, Zhou X Y. The function of uterine UDP-glucuronosyltransferase 1A8 (UGT1A8) and UDP-glucuronosyltransferase 2B7 (UGT2B7) is involved in endometrial cancer based on estrogen metabolism regulation. Hormones (Athens, Greece), 2020, 19(3): 403–412
Tian X G, Yan F, Zheng J Y, Cui X L, Feng L, Li S, Jin L L, James T D, Ma X C. Endoplasmic reticulum targeting ratiometric fluorescent probe for carboxylesterase 2 detection in drug-induced acute liver injury. Analytical Chemistry, 2019, 91(24): 15840–15845
Tian X G, Liu T, Li L, Shao B, Yao D H, Feng L, Cui J N, James T D, Ma X C. Visual high-throughput screening for developing a fatty acid amide hydrolase natural inhibitor based on an enzyme-activated fluorescent probe. Analytical Chemistry, 2020, 92(14): 9493–9500
Wang Y, Yu F B, Luo X Z, Li M S, Zhao L L, Yu F B. Visualization of carboxylesterase 2 with a near-infrared two-photon fluorescent probe and potential evaluation of its anticancer drug effects in an orthotopic colon carcinoma mice model. Chemical Communications, 2020, 56(32): 4412–4415
Yang Y, Zhou T T, Jin M, Zhou K Y, Liu D D, Li X, Huo F J, Li W, Yin C X. Thiol-chromene “click” reaction triggered self-immolative for NIR visualization of thiol flux in physiology and pathology of living cells and mice. Journal of the American Chemical Society, 2020, 142(3): 1614–1620
Li H Y, Liu Y, Li X Y, Li X H, Ma H M. Design, synthesis and application of a dual-functional fluorescent probe for reactive oxygen species and viscosity. Spectrochimica Acta. Part A: Molecular and Biomolecular Spectroscopy, 2021, 246: 119059
Gurram B, Li M, Fan J L, Wang J Y, Peng X J. Near-infrared fluorescent probe for fast track of cyclooxygenase-2 in Golgi apparatus in cancer cells. Frontiers of Chemical Science and Engineering, 2020, 14(1): 41–52
Xu S Y, Sedgwick A C, Elfeky S A, Chen W B, Jones A S, Williams G T, Jenkins A T A, Bull S D, Fossey J S, James T D. A boronic acid-based fluorescent hydrogel for monosaccharide detection. Frontiers of Chemical Science and Engineering, 2020, 14(1): 112–116
Liu T, Tian M M, Wang J Y, Tian X G, Liu J H, Feng L, Ma X C, Cui J N. Rational design of a fluorescent probe for the detection of LAP and its application in drug-induced liver injury. Spectrochimica Acta. Part A: Molecular and Biomolecular Spectroscopy, 2021, 251: 119362
Liu H W, Chen L L, Xu C Y, Li Z, Zhang H Y, Zhang X B, Tan W H. Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chemical Society Reviews, 2018, 47 (18): 7140–7180
Zhang J J, Chai X Z, He X P, Kim H J, Yoon J, Tian H. Fluorogenic probes for disease-relevant enzymes. Chemical Society Reviews, 2019, 48(2): 683–722
Wu X F, Shi W, Li X H, Ma H M. Recognition moieties of small molecular fluorescent probes for bioimaging of enzymes. Accounts of Chemical Research, 2019, 52(7): 1892–1904
Ning J, Liu T, Dong P P, Wang W, Ge G B, Wang B, Yu Z L, Shi L, Tian X G, Huo X K, et al. Molecular design strategy to construct the near-infrared fluorescent probe for selectively sensing human cytochrome P450 2J2. Journal of the American Chemical Society, 2019, 141(2): 1126–1134
Liu Z P, Sun Q. A near-infrared fluorescent probe for imaging of nitroxyl in living cells. Spectrochimica Acta. Part A: Molecular and Biomolecular Spectroscopy, 2020, 241: 118680
Ning J, Wang W, Ge G B, Chu P, Long F D, Yang Y L, Peng Y L, Feng L, Ma X C, James T D. Target enzyme-activated two-photon fluorescent probes: a case study of CYP3A4 using a two-dimensional design strategy. Angewandte Chemie International Edition, 2019, 58(29): 9959–9963
Feng L, Ning J, Tian X G, Wang C, Zhang L, Ma X C, James T D. Fluorescent probes for bioactive detection and imaging of phase II metabolic enzymes. Coordination Chemistry Reviews, 2019, 399: 213026
Ma H M. Spectroscopic Probes and Sensing Analysis. Beijing: Chemical Industry Press, 2020 (in Chinese)
Feng L, Ning J, Tian X G, Wang C, Yu Z L, Huo X K, Xie T, Zhang B J, James T D, Ma X C. Fluorescent probes for the detection and imaging of cytochrome P450. Coordination Chemistry Reviews, 2021, 437: 213740
Feng L, Tian Z H, Zhang M, He X, Tian X G, Yu Z L, Ma X C, Wang C. Real-time identification of gut microbiota with amino-peptidase N using an activable NIR fluorescent probe. Chinese Chemical Letters, 2021, https://doi.org/10.1016/j.cclet.2021.03.056
Terai T, Tomiyasu R, Ota T, Ueno T, Komatsu T, Hanaoka K, Urano Y, Nagano T. TokyoGreen derivatives as specific and practical fluorescent probes for UDP-glucuronosyltransferase (UGT) 1A1. Chemical Communications, 2013, 49(30): 3101–3103
Lv X, Feng L, Ai C Z, Hou J, Wang P, Zou L W, Cheng J, Ge G B, Cui J N, Yang L. A practical and high-affinity fluorescent probe for uridine diphosphate glucuronosyltransferase 1A1: a good surrogate for bilirubin. Journal of Medicinal Chemistry, 2017, 60(23): 9664–9675
Kim B, Fukuda M, Lee J Y, Su D, Sanu S, Silvin A, Khoo A T T, Kwon T, Liu X, Chi W, Liu X, Choi S, Wan D S Y, Park S J, Kim J S, Ginhoux F, Je H S, Chang Y T. Visualizing microglia with a fluorescence turn-on Ugt1a7c substrate. Angewandte Chemie International Edition, 2019, 58(24): 7972–7976
Lee J S, Kang N Y, Kim Y K, Samanta A, Feng S, Kim H K, Vendrell M, Park J H, Chang Y T. Synthesis of a BODIPY library and its application to the development of live cell glucagon imaging probe. Journal of the American Chemical Society, 2009, 131(29): 10077–10082
Huang Y P, Cao Y F, Fang Z Z, Zhang Y Y, Hu C M, Sun X Y, Yu Z W, Zhu X, Hong M, Yang L, Sun H Z. Glycyrrhetinic acid exhibits strong inhibitory effects towards UDP-glucuronosyltransferase (UGT) 1A3 and 2B7. Phytotherapy Research, 2013, 27(9): 1358–1361
Zhu L L, Ge G B, Liu Y, He G Y, Liang S C, Fang Z Z, Dong P P, Cao Y F, Yang L. Potent and selective inhibition of magnolol on catalytic activities of UGT1A7 and 1A9. Xenobiotica, 2012, 42(10): 1001–1008
He Y Q, Liu Y, Zhang B F, Liu H X, Lu Y L, Yang L, Xiong A Z, Xu L L, Wang C H, Yang L, Wang Z T. Identification of the UDP-glucuronosyltransferase isozyme involved in senecionine glucuronidation in human liver microsomes. Drug Metabolism and Disposition: the Biological Fate of Chemicals, 2010, 38(4): 626–634
Sahai J, Gallicano K, Pakuts A, Cameron D W. Effect of fluconazole on zidovudine pharmacokinetics in patients infected with human immunodeficiency virus. Journal of Infectious Diseases, 1994, 169 (5): 1103–1107
Yan F, Cui Y L, An Y, Ning J, Zhao X Y, Feng L, Huo X K, Wang C, Lv C Z, Ma X C, Tian X. A dual functional probe for assessing human CYP450 3A5 and 3A enzymes bioactivities. Future Medicinal Chemistry, 2019, 11(22): 2891–2903
Tian X G, Huo X K, Dong P P, Wu B J, Wang X B, Wang C, Liu K X, Ma X C. Sulfation of melatonin: enzymatic characterization, differences of organs, species and genders, and bioactivity variation. Biochemical Pharmacology, 2015, 94(4): 282–296
Acknowledgments
The authors thank the Natural Science Foundation of Liaoning Province 2020-MS-252 and the National Key R&D Program of China (Grant No. 2018YFC1603001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Declaration of competing interest All the authors declare no competing financial interest.
Electronic Supplementary Material
11705_2021_2064_MOESM1_ESM.pdf
A highly selective fluorescent probe for real-time imaging of UDP-glucuronosyltransferase 1A8 in living cells and tissues
Rights and permissions
About this article
Cite this article
Zhu, M., Tian, Z., Jin, L. et al. A highly selective fluorescent probe for real-time imaging of UDP-glucuronosyltransferase 1A8 in living cells and tissues. Front. Chem. Sci. Eng. 16, 103–111 (2022). https://doi.org/10.1007/s11705-021-2064-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-021-2064-8