Abstract
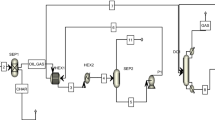
New and efficient reactor systems were proposed to treat perfluorinated compounds via catalytic decomposition. One system has a single reactor (S-1), and another has a series of reactors (S-2). Both systems are capable of producing a valuable CaF2 and eliminating toxic HF effluent and their feasibility was studied at various temperatures with a commercial process simulator, Aspen HYSYS®. They are better than the conventional system, and S-2 is better than S-1 in terms of CaF2 production, a required heat for the system, natural gas usage and CO2 emissions in a boiler, and energy consumption. Based on process simulation results, preliminary economic analysis shows that cost savings of 12.37% and 13.55% were obtained in S-2 at 589.6 and 621.4 °C compared to S-1 at 700 and 750 °C, respectively, for the same amount of CaF2 production.

Similar content being viewed by others
References
Gompel J V, Walling T. A new way to treat process exhaust to remove CF4. Semiconductor International, 1977, 20(10): 95–100
Tsai W T, Chem H P, Hsien W Y. A review of uses, environmental hazards and recovery/recycle technologies of perfluorocarbons (PFCs) emissions from the semiconductor manufacturing processes. Journal of Loss Prevention in the Process Industries, 2002, 15(2): 65–75
Brown R S, Rossin J A, Thomas C J. Catalytic process for control of PFC emissions. Semiconductor International, 2001, 24(6): 209–215
Takita Y, Morita C, Ninomiya M, Wakamatsu H, Nishiguchi H, Ishihara T. Catalytic decomposition of CF4 over AlPO4-based catalysts. Chemistry Letters, 1999, 28(5): 417–418
Xu X F, Jeon J Y, Choi M H, Kim H Y, Choi W C, Park Y K. A strategy to protect Al2O3-based PFC decomposition catalyst from deactivation. Chemistry Letters, 2005, 34(3): 364–365
Song J, Chung S, Kim M, Seo M, Lee Y, Lee K, Kim J. The catalytic decomposition of CF4 over Ce/Al2O3 modified by a cerium sulfate precursor. Journal of Molecular Catalysis A Chemical, 2013, 370: 50–55
Han W, Chen Y, Kin B, Liu H, Yu H. Catalytic hydrolysis of trifluoromethane over alumina. Greenhouse Gases. Science and Technology, 2014, 4(1): 121–130
Park N, Park H, Lee T, Chang W, Kwon W. Hydrolysis and oxidation on supported phosphate catalyst for decomposition of SF6. Catalysis Today, 2012, 185(1): 247–252
Lee Y C, Jeon J K. A study on catalytic process in pilot plant for abatement of PFC emission. Cleanroom Technology, 2012, 18(2): 216–220
Elkanzi E M. Simulation of the process of biological removal of hydrogen sulfide from gas. In: Proceedings of the 1st Annual Gas Processing Symposium, 2009, 1: 266–275
Sunny A, Solomon P A, Aparna K. Syngas production from regasified liquefied natural gas and its simulation using Aspen HYSYS. Journal of Natural Gas Science and Engineering, 2016, 30: 176–181
Kazemi A, Malayeri M, Gharibi kharaji A, Shariati A. Gharibi kharaji A, Shariati A. Feasibility study, simulation, and economical evaluation of natural gas sweetening processes–Part 1: A case study on a low capacity plant in iran. Journal of Natural Gas Science and Engineering, 2014, 20: 16–22
Peters L, Hussain A, Follmann M, Melin T, Hagg M B. CO2 removal from natural gas by employing amine absorption and membrane technology–A technical and economical analysis. Chemical Engineering Journal, 2011, 172(2-3): 952–960
Ahmad F, Lau K K, Shariff A M, Murshid G. Process simulation and optimal design of membrane separation system for CO2 capture from natural gas. Computers & Chemical Engineering, 2012, 36(10): 119–128
Ploegmakers J, Jelsma A R T, van der Ham A G J, Nijmeijer K. Economic evaluation of membrane potential for ethylene/ethane separation in a retrofitted hybrid membrane-distillation plant using Unisim Design. Industrial & Engineering Chemistry Research, 2013, 52(19): 6524–6539
Choi J, Park M, Kim J, Ko Y, Lee S, Baek I. Modelling and analysis of pre-combustion CO2 capture with membranes. Korean Journal of Chemical Engineering, 2013, 30(6): 1187–1194
Marchioro Y P A, Lakew A A, Bolland O. Integration of lowtemperature transcritical CO2 Rankine cycle in natural gas-fired combined cycle (NGCC) with post-combustion CO2 capture. International Journal of Greenhouse Gas Control, 2013, 12: 213–219
Park M, Kim E. Thermodynamic evaluation on the integrated system of VHTR and forward osmosis desalination process. Desalination, 2014, 337(17): 117–126
Nahar G A, Madhani S S. Thermodynamics of hydrogen production by the steam reforming of butanol: Analysis of inorganic gases and light hydrocarbons. International Journal of Hydrogen Energy, 2010, 35(1): 98–109
Leonzio G. Process analysis of biological Sabatier reaction for biomethane production. Chemical Engineering Journal, 2016, 290(15): 490–498
Denz N, Ausberg L, Bruns M, Viere T. Supporting resource efficiency in chemical industries IT-based integration of flow sheet simulation and material flow analysis. 21st CIRP Conference on Life Cycle Engineering, 2014, 15: 537–542
Ou L, Thilakaratne R, Brown R C, Wright M M. Techno-economic analysis of transportation fuels from defatted microalgae via hydrothermal liquefaction and hydroprocessing. Biomass and Bioenergy, 2015, 72: 45–54
Apostolakou A A, Kookos I K, Marazioti C, Angelopoulos K C. Techno-economic analysis of a biodiesel production process from vegetable oils. Fuel Processing Technology, 2009, 90(7-8): 1023–1031
Sanchez M J G, Tsotsis T T. Catalytic membranes and membrane reactors. Weinheim: Wiley-VCH, 2002, 5–6
Lim H. Hydrogen selectivity and permeance effect on the water gas shift reaction (WGSR) in a membrane reactor. Korean Journal of Chemical Engineering, 2015, 32(8): 1522–1527
Turton R. Analysis, synthesis, and design of chemical processes. New Jersey: Pearson, 2013, 157–226
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lee, B., Lee, S., Jung, H.Y. et al. Process simulation and economic analysis of reactor systems for perfluorinated compounds abatement without HF effluent. Front. Chem. Sci. Eng. 10, 526–533 (2016). https://doi.org/10.1007/s11705-016-1590-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-016-1590-2