Abstract
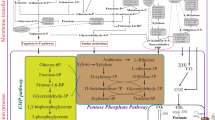
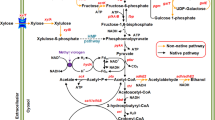
Butanol is a promising biofuel with high energy intensity and can be used as gasoline substitute. It can be produced as a sustainable energy by microorganisms (such as Clostridia) from low-value biomass. However, the low productivity, yield and selectivity in butanol fermentation are still big challenges due to the lack of an efficient butanol-producing host strain. In this article, we systematically review the host cell engineering of Clostridia, focusing on (1) various strategies to rebalance metabolic flux to achieve a high butanol production by regulating the metabolism of carbon, redox or energy, (2) the challenges in pathway manipulation, and (3) the application of proteomics technology to understand the intracellular metabolism. In addition, the process engineering is also briefly described. The objective of this review is to summarize the previous research achievements in the metabolic engineering of Clostridium and provide guidance for future novel strain construction to effectively produce butanol.

Similar content being viewed by others
References
Dürre P. Fermentative butanol production. Annals of the New York Academy of Sciences. 2008, 1125(1): 353–362
Caspeta L, Buijs N A A, Nielsen J. The role of biofuels in the future energy supply. Energy & Environmental Science. 2013, 6(4): 177–182
Lütke-Eversloh T, Bahl H. Metabolic engineering of Clostridium acetobutylicum: Recent advances to improve butanol production. Current Opinion in Biotechnology. 2011, 22(5): 634–647
Tracy B P, Jones S W, Fast A G, Indurthi D C, Papoutsakis E T. Clostridia: The importance of their exceptional substrate and metabolite diversity for biofuel and biorefinery applications. Current Opinion in Biotechnology. 2012, 23(3): 364–381
Zheng Y, Li L, Xian M, Ma Y, Yang J, Xu X, He D. Problems with the microbial production of butanol. Journal of Industrial Microbiology & Biotechnology. 2009, 36(9): 1127–1138
Yu M, Zhang Y, Tang I, Yang S. Metabolic engineering of Clostridium tyrobutyricum for n-butanol production. Metabolic Engineering. 2011, 13(4): 373–382
Harris L M, Desai R P, Welker N E, Papoutsakis E T. Characterization of recombinant strains of the Clostridium acetobutylicum butyrate kinase inactivation mutant: Need for new phenomenological models for solventogenesis and butanol inhibition? Biotechnology and Bioengineering, 2000, 67(1): 1–11
Jang Y S, Lee J Y, Lee J, Park J H, Im J A, Eom M H, Lee J, Lee S H, Song H, Cho J H, Seung D Y, Lee S Y. Enhanced butanol production obtained by reinforcing the direct butanol-forming route in Clostridium acetobutylicum. mBio. 2012, 3(5): 1–9
Cooksley C M, Zhang Y, Wang H, Redl S, Winzer K, Minton N P. Targeted mutagenesis of the Clostridium acetobutylicum acetonebutanol- ethanol fermentation pathway. Metabolic Engineering. 2012, 14(6): 630–641
Cai G, Jin B, Saint C, Monis P. Genetic manipulation of butyrate formation pathways in Clostridium butyricum. Journal of Biotechnology. 2011, 155(3): 269–274
Lu C. Butanol production from lignocellulosic feedstocks by acetone-butanol-ethanol fermentation with integrated product recovery. Dissertation for the Doctoral Degree. Columbus: The Ohio State University. 2011, 2–25
Tummala S B, Welker N E, Papoutsakis E T. Design of antisense RNA constructs for downregulation of the acetone formation pathway of Clostridium acetobutylicum. Journal of Bacteriology. 2003, 185(6): 1923–1934
Nair R V, Papoutsakis E T. Expression of plasmid-encoded aad in Clostridium acetobutylicum M5 restores vigorous butanol production. Journal of Bacteriology. 1994, 176(18): 5843–5846
Yu L, Zhao J, Xu M, Dong J, Varghese S, Yu M, Tang I, Yang S. Metabolic engineering of Clostridium tyrobutyricum for n-butanol production: Effects of CoA transferase. Applied Microbiology and Biotechnology. 2015, published online
Ma C, Kojima K, Xu N, Mobley J, Zhou L, Yang S, Liu X M. Comparative proteomics analysis of high n-butanol producing metabolically engineered Clostridium tyrobutyricum. Journal of Biotechnology. 2015, 193: 108–119
Rajagopalan G, He J, Yang K. A highly efficient NADH-dependent butanol dehydrogenase from high-butanol-producing Clostridium sp. BOH3. BioEnergy Research. 2013, 6(1): 240–251
Kuit W, Minton N P, López-Contreras A M, Eggink G. Disruption of the acetate kinase (ack) gene of Clostridium acetobutylicum results in delayed acetate production. Applied Microbiology and Biotechnology. 2012, 94(3): 729–741
Zhang Y, Yu M, Yang S. Effects of ptb knockout on butyric acid fermentation by Clostridium tyrobutyricum. Biotechnology Progress. 2012, 28(1): 52–59
Lehmann D, Radomski N, Lütke-Eversloh T. New insights into the butyric acid metabolism of Clostridium acetobutylicum. Applied Microbiology and Biotechnology. 2012, 96(5): 1325–1339
Heap J T, Cartman S T, Pennington O J, Cooksley C M, Scott J C, Blount B, Burns D A, Minton N P. Clostridia: Molecular Biology in the Post-genomic Era. Norfolk: Caister Academic Press. 2009, 179–198
Heap J T, Pennington O J, Cartman S T, Carter G P, Minton N P. The ClosTron: A universal gene knock-out system for the genus Clostridium. Journal of Microbiological Methods. 2007, 70(3): 452–464
Heap J T, Kuehne S A, Ehsaan M, Cartman S T, Cooksley C M, Scott J C, Minton N P. The ClosTron: Mutagenesis in Clostridium refined and streamlined. Journal of Microbiological Methods. 2010, 80(1): 49–55
Durre P, Bohringer M, Nakotte S, Schaffer S, Thormann K, Zickner B. Transcriptional regulation of solventogenesis in Clostridium acetobutylicum. Journal of Molecular Microbiology and Biotechnology. 2002, 4(3): 295–300
Lee S Y, Park J H, Jang S H, Nielsen L K, Kim J, Jung K S. Fermentative butanol production by clostridia. Biotechnology and Bioengineering. 2008, 101(2): 209–228
Jang Y, Han M, Lee J, Im J A, Lee Y H, Papoutsakis E T, Bennett G, Lee S Y. Proteomic analyses of the phase transition from acidogenesis to solventogenesis using solventogenic and nonsolventogenic Clostridium acetobutylicum strains. Applied Microbiology and Biotechnology. 2014, 98(11): 5105–5115
Yang S, Giannone R J, Dice L, Yang Z K, Engle N L, Tschaplinski T J, Hettich R L, Brown S D. Clostridium thermocellum ATCC27405 transcriptomic, metabolomic and proteomic profiles after ethanol stress. BMC Genomics. 2012, 13(1): 336–353
Nakayama S, Kosaka T, Hirakawa H, Matsuura K, Yoshino S, Furukawa K. Metabolic engineering for solvent productivity by downregulation of the hydrogenase gene cluster hupCBA in Clostridium saccharoperbutylacetonicum strain N1-4. Applied Microbiology and Biotechnology. 2008, 78(3): 483–493
Inui M, Suda M, Kimura S, Yasuda K, Suzuki H, Toda H, Yamamoto S, Okino S, Suzuki N, Yukawa H. Expression of Clostridium acetobutylicum butanol synthetic genes in Escherichia coli. Applied Microbiology and Biotechnology. 2008, 77(6): 1305–1316
Grupe H, Gottschalk G. Physiological events in Clostridium acetobutylicum during the shift from acidogenesis to solventogenesis in continuous culture and presentation of a model for shift induction. Applied and Environmental Microbiology. 1992, 58(12): 3896–3902
Berríos-Rivera S. The effect of increasing NADH availability on the redistribution of metabolic fluxes in Escherichia coli chemostat cultures. Metabolic Engineering. 2002, 4(3): 230–237
Vemuri G N, Eiteman M A, McEwen J E, Olsson L, Nielsen J. Increasing NADH oxidation reduces overflow metabolism in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America. 2007, 104(7): 2402–2407
Jo J H, Lee D S, Park J M. The effects of pH on carbon material and energy balances in hydrogen-producing Clostridium tyrobutyricum JM1. Bioresource Technology. 2008, 99(17): 8485–8491
Jiang M, Chen J, He A, Wu H, Kong X, Liu J, Yin C, Chen W, Chen P. Enhanced acetone/butanol/ethanol production by Clostridium beijerinckii IB4 using pH control strategy. Process Biochemistry. 2014, 49(8): 1238–1244
Tsai T, Lo Y, Chang J. Effect of medium composition and pH control strategies on butanol fermentation with Clostridium acetobutylicum. Energy Procedia. 2014, 61: 1691–1694
Vardar-Schara G, Maeda T, Wood T K. Metabolically engineered bacteria for producing hydrogen via fermentation. Microbial Biotechnology. 2008, 1(2): 107–125
Li F, Hinderberger J, Seedorf H, Zhang J, Buckel W, Thauer R K. Coupled ferredoxin and crotonyl coenzyme A (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from Clostridium kluyveri. Journal of Bacteriology. 2008, 190(3): 843–850
Shen C, Lan E, Dekishima Y, Baez A, Cho K M, Liao J. Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli. Applied and Environmental Microbiology. 2011, 77(9): 2905–2915
Yu R, Wang R, Bi T, Sun W, Zhou Z. Blocking the butyrateformation pathway impairs hydrogen production in Clostridium perfringens. Acta Biochimica et Biophysica Sinica. 2013, 45(5): 408–415
Wang Y, San K Y, Bennett G N. Cofactor engineering for advancing chemical biotechnology. Current Opinion in Biotechnology. 2013, 24(6): 994–999
Du Y, Jiang W, Yu M, Tang I, Yang S. Metabolic process engineering of Clostridium tyrobutyricum Δack-adhE2 for enhanced n-butanol production from glucose: Effects of methyl viologen on NADH availability, flux distribution, and fermentation kinetics. Biotechnology and Bioengineering. 2015, 112(4): 705–715
Ujor V, Agu C V, Gopalan V, Ezeji T C. Glycerol supplementation of the growth medium enhances in situ detoxification of furfural by Clostridium beijerinckii during butanol fermentation. Applied Microbiology and Biotechnology. 2014, 98(14): 6511–6521
Hüsemann M H, Papoutsakis E T. Comparison between in vivo and in vitro enzyme activities in continuous and batch fermentations of Clostridium acetobutylicum. Applied Microbiology and Biotechnology. 1989, 30(6): 585–595
Wiesenborn D P, Rudolph F B, Papoutsakis E T. Thiolase from Clostridium acetobutylicum ATCC 824 and its role in the synthesis of acids and solvents. Applied and Environmental Microbiology. 1988, 54(11): 2717–2722
Lan E, Liao J. ATP drives direct photosynthetic production of 1-butanol in cyanobacteria. Proceedings of the National Academy of Sciences of the United States of America. 2012, 109(16): 6018–6023
Ventura J S, Hu H, Jahng D. Enhanced butanol production in Clostridium acetobutylicum ATCC 824 by double overexpression of 6-phosphofructokinase and pyruvate kinase genes. Applied Microbiology and Biotechnology. 2013, 97(16): 7505–7516
Bowles L K, Ellefson W L. Effects of butanol on Clostridium acetobutylicum. Applied and Environmental Microbiology. 1985, 50(5): 1165–1170
Wang J, Yang X, Chen C, Yang S. Engineering clostridia for butanol production from biorenewable resources: from cells to process integration. Current Opinion in Chemical Engineering. 2014, 6: 43–54
Jo J H, Lee D S, Kim J, Park J M. Effect of initial glucose concentrations on carbon material and energy balances in hydrogenproducing Clostridium tyrobutyricum JM1. Journal of Microbiology and Biotechnology. 2009, 19(3): 291–298
Herrmann G, Jayamani E, Mai G, Buckel W. Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. Journal of Bacteriology. 2008, 190(3): 784–791
Lan E, Liao J. Microbial synthesis of n-butanol, isobutanol, and other higher alcohols from diverse resources. Bioresource Technology. 2013, 135: 339–349
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the 120th Anniversary of Tianjin University
Rights and permissions
About this article
Cite this article
Ou, J., Ma, C., Xu, N. et al. High butanol production by regulating carbon, redox and energy in Clostridia. Front. Chem. Sci. Eng. 9, 317–323 (2015). https://doi.org/10.1007/s11705-015-1522-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-015-1522-6