Abstract
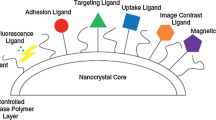
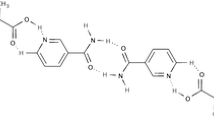
The applications of the crystallization technique in the pharmaceutical industry as a purification and separation process for the isolation and synthesis of pure active pharmaceutical ingredients (API), co-crystals, controlled release pulmonary drug delivery, and separation of chiral isomers are briefly discussed using a few case studies. The effect of process variables and solvent on the polymorphism and morphology of stavudine is discussed. The implementation of external control in the form of feedback and real-time optimal control using cooling and antisolvent crystallization of paracetamol in water-isopropyl alcohol is introduced. Two methods to prepare micronsized drug particles, namely, micro-crystallization and polymer-coated API-loaded magnetic nanoparticles for pulmonary drug delivery, are discussed. The significance of co-crystals in drug administration is highlighted using the theophylline-nicotinamide co-crystal system. Resolution of chloromandelic acid derivatives, a racemic compound, is achieved using direct crystallization and diastereomeric salts crystallization. The crystal structures of diastereomeric salts of chloromandelic acid and phenylethylamine are determined. The structure comparison between the less soluble and more soluble salts shows that weak interactions such as CH/π interactions and van der Waals forces contribute to chiral recognition when the hydrogen bonding patterns are similar.
Similar content being viewed by others
References
McKie D, McKie C. Essentials of Crystallography. London: Blackwell Scientific Publications, 1986
Mullin J. Crystallization. 3rd ed. London: Butterworth-Heinemann, 1993
Rohani S, Horne S, Murthy K S K. Control of the product quality in batch crystallization of pharmaceuticals and fine chemicals. Part 1: design of the crystallization process and the effect of solvent. Org Res Proc Dev, 2005, 9(6): 858–872
Rohani S, Horne S, Murthy K S K. Control of the product quality in batch crystallization of pharmaceuticals and fine chemicals. Part 2: external control. Org Res Proc Dev, 2005, 9(6): 873–883
Mirmehrabi M, Rohani S, Murthy K S K, Radatus B. Polymorphic behaviour and crystal habit of an anti-viral/HIV drug: stavudine. J Crystal Growth & Des, 2006, 6(1): 141–149
Skonezny P M, Eisenreich E, Stark D R, Boyhan B T, Baker S R. Process for large scale preparation of 2′,3′-didehydro-2′,3′-dideonucleosides. EP patent 0653435A1, 1995
Radatus B K, Murthy K S K. Process for the preparation of substantially pure stavudine and related intermediates useful in the preparation thereof. USA patent 6635753B1, 2003
Mirmehrabi M, Rohani S. An approach to solvent screening for crystallization of polymorphic pharmaceuticals and fine chemicals. J Pharm Sci, 2005, 94(7): 1560–1576
Lu J, Rohani S. Preparation and characterization of theophyllinenicotinamide co-crystal. Org Proc Res Dev, 2009, 13(6), 1269–1275
Mullin J W, Nyvlt J. Programmed cooling of batch crystallizers. Chem Eng Sci, 1971, 26: 369–377
Jones A G, Mullin J W. Programmed cooling crystallization of potassium sulphate solutions. Chem Eng Sci, 1974, 29, 105–118
Matthews H B, Rawlings J B. Batch crystallization of a photochemical: modeling, control, and filtration. AIChE J, 1998, 44: 1119–1127
Chung S H, Ma D L, Braatz R D. Optimal seeding in batch crystallization. Can J Chem Eng, 1999, 77: 590–596
Hu Q, Rohani S, Wang D X, Jutan A. Nonlinear kinetic parameter estimation for batch cooling seeded crystallization. AIChE J, 2004, 50(8): 1786–1794
Sheikhzadeh M, Trifkovic M, Rohani S. Adaptive MIMO neurofuzzy logic control of a seeded and an unseeded anti-solvent semibatch crystallizer, 2008. Chem Eng Sci, 63(5): 1261–1272
Zhang G, Rohani S. On-line optimal control of a seeded batch crystallizer. Chem Eng Sci, 2003, 58(9): 1887–1896
Trifkovic M, Sheikhzadeh M, Rohani S. Multivariable real-time optimal control of a cooling and anti-solvent semi-batch crystallization process, 2008. AIChE J, 2009, 55(10): 2591–2602
Ragab D, Rohani S, Samaha M W, El-Khawas F M, El-Maradny H A. Crystallization of progesterone for pulmonary drug delivery. J Pharm Sci, 2009, 09–087.R1
Sholl D S, Gellam A J. Developing chiral surfaces for enantioselective chemical processing. AIChE J 2009, 55(10): 2484–2490
Jacques J, Collet A, Wilen S H. Enantiomers, Racemates and Resolutions. New York: Wiley, 1981
He Q, Zhu J, Gomaa H, Jennings M, Rohani S. Identification and characterization of solid nature of 2-chloromandelic acid. J Pharm Sci, 2009, 98(5): 1835–1844
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rohani, S. Applications of the crystallization process in the pharmaceutical industry. Front. Chem. Eng. China 4, 2–9 (2010). https://doi.org/10.1007/s11705-009-0297-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-009-0297-z