Abstract
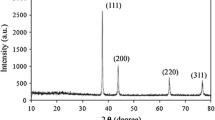
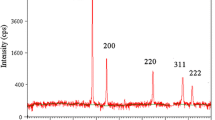
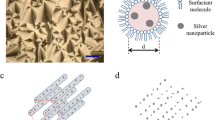
A simple solution-phase approach has been demonstrated for the large-scale synthesis of silver nanowires with diameters in the range of 15–25 nm, and lengths usually in the range of tens of micrometers. In the presence of gemini surfactant 1,3-bis(cetyldimethylammonium) propane dibromide (16-3-16), the growth of silver could be directed into a highly anisotropic mode to form uniform nanowires with aspect ratios up to about 2,000. X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), energy-dispersive X-ray (EDX), X-ray powder diffraction (XRD), electron diffraction (ED), and UV-vis absorption spectroscopy, were used to characterize the as-prepared silver nanowires, indicating the formation of a highly pure phase, good crystallinity, as well as a uniform diameter.
Similar content being viewed by others
References
El-Sayed M A. Some interesting properties of metals confined in time and nanometer space of different shapes. Accounts Chem Res, 2001, 34: 257–264
Peng X G, Manna L, Yang W D, Wickham J, Scher E, Kadavanich A, Alivisatos A P. Shape control of CdSe nanocrystals. Nature, 2000, 404: 59–61
Lieber C M. One-dimensional nanostructures: Chemistry, physics and applications. Solid State Commun, 1998, 107: 607–616
Templeton A C, Wuelfing W P, Murray R W. Monolayer-protected cluster molecules. Accounts Chem Res, 2000, 33: 27–36
Zhou K B, Wang X, Sun X M, Peng Q, Li Y D. Enhanced catalytic activity of ceria nanorods from well-defined reactive crystal planes. J Catal, 2005, 229: 206–212
Xu J, Han X, Liu H, Hu Y. Synthesis and optical properties of silver nanoparticles stabilized by gemini surfactant. Colloid Surface A, 2006, 273: 179–183
Jin R C, Cao Y W, Mirkin C A, Kelly K L, Schatz G C, Zheng J G. Photoinduced conversion of silver nanospheres to nanoprisms. Science, 2001, 294: 1,901–1,903
Jiang P, Li S Y, Xie S S, Gao Y, Song L. Machinable long PVP-stabilized silver nanowires. Chem-Eur J, 2004, 10: 4,817–4,821
Wang Z H, Liu J W, Chen X Y, Wan J X, Qian Y T. A simple hydrothermal route to large-scale synthesis of uniform silver nanowires. Chem-Eur J, 2005, 11: 160–163
Wiley B, Sun Y G, Mayers B, Xia Y N. Shape-controlled synthesis of metal nanostructures: The case of silver. Chem-Eur J, 2005, 11: 454–463
Wang W, Huang J, Ren Z. Synthesis of germanium nanocubes by a low-temperature inverse micelle solvothermal technique. Langmuir, 2005, 21: 751–754
Feng J, Zeng H C. Size-controlled growth of Co3O4 nanocubes. Chem Mater, 2003, 15: 2,829–2,835
Sau T K, Murphy C J. Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J Am Chem Soc, 2004, 126: 8,648–8,649
Xu R, Zeng H C. Mechanistic investigation on salt-mediated formation of free-standing Co3O4 nanocubes at 95°C. J Phys Chem B, 2003, 107: 926–930
Xiong Y, Wiley B, Chen J, Li Z Y, Yin Y, Xia Y N. Corrosion-based synthesis of single-crystal Pd nanoboxes and nanocages and their surface plasmon properties. Angew Chem Int Edit, 2005, 44: 7,913–7,917
Sun X, Li Y. Ga2O3 and GaN semiconductor hollow spheres. Angew Chem Int Edit, 2004, 43: 3,827–3,831
Kong X Y, Ding Y, Wang Z L. Metal-semiconductor Zn-ZnO core-shell nanobelts and nanotubes. J Phys Chem B, 2004, 108: 570–574
Zheng X, Zhu L, Yan A, Wang X, Xie Y. Controlling synthesis of silver nanowires and dendrites in mixed surfactant solutions. J Colloid Interf Sci, 2003, 268: 357–361
Ma Y, Qi L, Shen W, Ma J. Selective synthesis of single-crystalline selenium nanobelts and nanowires in micellar solutions of nonionic surfactants. Langmuir, 2005, 21: 6,161–6,164
Wang X, Li Y. Selected-control hydrothermal synthesis of α-and β-MnO2 single crystal nanowires. J Am Chem Soc, 2002, 124: 2,880–2,881
Ni C, Hassan P A, Kaler E W. Structural characteristics and growth of pentagonal silver nanorods prepared by a surfactant method. Langmuir, 2005, 21: 3,334–3,337
Favie F, Walter E C, Zach M P, Benter T, Penner R M. Hydrogen sensors and switches from electroeposited palladium mesowire arrays. Science, 2001, 293: 2,227–2,231
Cui Y, Wei Q Q, Park H K, Lieber C M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science, 2001, 293: 1,289–1,292
Gudiksen M S, Lauhon L J, Wang J, Smith D C, Lieber C M. Growth of nanowire superlattice structures for nanoscale photonics and electronics. Nature, 2002, 415: 617–620
Hu J T, Odom T W, Lieber C M. Chemistry and physics in one dimension: Synthesis and properties of nanowires and nanotubes. Accounts Chem Res, 1999, 32, 435–445
Sun L, Searson P C, Chien C L. Magnetic anisotropy in prismatic nickel nanowires. Appl Phys Lett, 2001, 79: 4,429–4,431
Peng X G. Mechanisms for the shape-control and shape-evolution of colloidal semiconductor nanocrystals. Adv Mater, 2003, 15: 459–463
Zong R L, Zhou J, Li Q, Du B, Li B, Fu M, Qi X W, Li L T, Buddhudu S. Synthesis and optical properties of silver nanowire arrays embedded in anodic alumina membrane. J Phys Chem B, 2004, 108: 16,713–16,716
Choi J, Sauer G, Nielsch K, Wehrspohn R B, Gosele U. Hexagonally arranged monodisperse silver nanowires with adjustable diameter and high aspect ratio. Chem Mater, 2003, 15: 776–779
Wu Y, Livneh T, Zhang Y X, Cheng G, Wang J, Tang J, Moskovits M, Stucky G D. Templated synthesis of highly ordered mesostructured nanowires and nanowire arrays. Nano Lett, 2004, 4: 2,337–2,342
Day T M, Unwin P R, Wilson N R, Macpherson J V. Electrochemical templating of metal nanoparticles and nanowires on single-walled carbon nanotube networks. J Am Chem Soc, 2005, 127: 10,639–10,647
Braun E, Eichen Y, Sivan U, Ben-Yoseph G. DNA-templated assembly and electrode attachment of a conducting silver wire. Nature, 1998, 391: 775–778
Zhang D, Qi L, Ma J, Cheng H. Formation of silver nanowires in aqueous solutions of a double-hydrophilic block copolymer. Chem Mater, 2001, 13: 2,753–2,755
Jana N R, Gearheart L, Murphy C J. Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio. Chem Commun, 2001, 617–618
Zhou Y, Yu S H, Wang C Y, Li X G, Zhu Y R, Chen Z Y. A novel ultraviolet irradiation photoreduction technique for the preparation of single-crystal Ag nanorods and Ag dendrites. Adv Mater, 1999, 11: 850–852
Wang C, Chen M, Zhu G, Lin Z. A novel soft-template technique to synthesize metal Ag nanowire. J Colloid Interf Sci, 2001, 243: 362–364
Zhou Y, Yu S H, Cui X P, Wang C Y, Chen Z Y. Formation of silver nanowires by a novel solid-liquid phase are discharge method. Chem Mater, 1999, 11: 545–546
Zhu J J, Liu S W, Palchik O, Koltypin Y, Gedanken A. Shape-controlled synthesis of silver nanoparticles by pulse sonoelectrochemical methods. Langmuir, 2000, 16: 6,396–6,399
Xu J, Hu J, Peng C, Liu H, Hu Y. A simple approach to the synthesis of silver nanowires by hydrothermal process in the presence of gemini surfactant. J Colloid Interf Sci, 2006, 298: 689–693
Chen Q B, Wei Y H, Shi Y H, Liu H L, Hu Y. Measurement of surface tension and electrical conductivity of cationic gemini surfactants. Journal of East China University of Science and Technology, 2003, 29: 33–37 (in Chinese)
Gao Y, Jiang P, Liu D F, Yuan H J, Yan X Q, Zhou Z P, Wang J X, Song L, Liu L F, Zhou W Y, Wang G, Wang C Y, Xie S S. Evidence for the monolayer assembly of poly(vinylpyrrolidone) on the surfaces of silver nanowires. J Phys Chem B, 2004, 108: 12,877–12,881
Sun Y G, Mayers B, Herricks T, Xia Y N. Polyol synthesis of uniform silver nanowires: A plausible growth mechanism and the supporting evidence. Nano Lett, 2003, 3: 955–960
Sun Y G, Yin Y D, Mayers B T, Herricks T, Xia Y N. Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly(vinylpyrrolidone). Chem Mater, 2002, 14: 4,736–4,745
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., Liu, W., Liu, H. et al. Controlled synthesis of uniform silver nanowires with high aspect ratios in aqueous solutions of gemini surfactant. Front. Chem. Eng. China 1, 221–227 (2007). https://doi.org/10.1007/s11705-007-0040-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11705-007-0040-6