Abstract
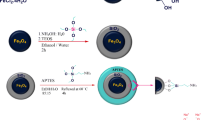
This work describes fabrication of Fe3O4@SiO2 core–shell nanoparticles functionalized with polyvinyl alcohol (PVA) and its application for the removal of Cu(П) and Cd(П) ions. Surface chemistry and morphology during functionalization of Fe3O4@SiO2 NPs were evaluated utilizing Fourier transform infrared spectroscopy, X-ray diffraction, energy-dispersive X-ray analysis, vibration sample magnetometer, transmission electron microscope, field emission scanning electron microscope, and dynamic light scattering. The performance of the PVA-modified magnetic nanoparticles for Cu(П) and Cd(П) ions removal was evaluated by changing five process variables: contact time of adsorbent, temperature, pH, adsorbent dosage, and initial concentration. The highest removal efficiency was observed at a pH value of 7, an adsorbent dose of 15 mg, an initial concentration of 0.5 mmol/L, and a contact time of 25 min for Cu(II), while these values are 7, 15 mg, 0.4 mmol/L, and 20 min for Cd(II), respectively. The adsorption equilibrium is well explained by Langmuir adsorption isotherms which namely monolayer adsorption. The adsorption isotherm studies of the novel adsorbent in removing target ions from wastewater showed that the maximum adsorption amounts of Cu(П) and Cd(П) were 1.57 and 0.99 mmol/g at 35 °C. According to the calculated parameters and fitting model, the kinetics adsorption data are well fitted to the pseudo-second-order kinetic model which suggests that the main rate-determining step was chemisorption’s. According to the results, the prepared composite possesses excellent adsorption efficiency to be applied for the preconcentration of target heavy metal ions in the field of water treatment. The new adsorbent is completely separated from the aqueous solution under external magnetic field for five consecutive cycles without significant loss of activity.








Similar content being viewed by others
References
Afshar MG, Crespo GA, Bakker E (2015) Thin-layer chemical modulations by a combined selective proton pump and pH probe for direct alkalinity detection. Angew Chem Int Ed 127:8228–8231
Ahmed HA, Soliman MS, Othman SA (2021) Synthesis and characterization of magnetic nickel ferrite-modified montmorillonite nanocomposite for cu (II) and zn (II) ions removal from wastewater, Egypt. J Chem 64:3–4
Ahmedzeki NS (2013) Adsorption filtration technology using iron-coated sand for the removal of lead and cadmium ions from aquatic solutions. Desalination Water Treat 51:5559–5565
Aliabadi M, Irani M, Ismaeili J, Piri H, Parnian MJ (2013) Electrospun nanofiber membrane of PEO/Chitosan for the adsorption of nickel, cadmium, lead and copper ions from aqueous solution. J Chem Eng 220:237–243
Asgharinezhad AA, Ebrahimzadeh H (2016) Poly (2-aminobenzothiazole)-coated graphene oxide/magnetite nanoparticles composite as an efficient sorbent for determination of non-steroidal anti-inflammatory drugs in urine sample. J Chromatogr A 1435:18–29
Asgharinezhad AA, Ebrahimzadeh H (2020) A novel polymer coated magnetic porous carbon nanocomposite derived from a metal-organic framework for multi-target environmental pollutants preconcentration. J Chromatogr A 1634:461664
Asgharinezhad AA, Ebrahimzadeh H (2021) Magnetic porous carbon nanocomposite derived from cobalt based-metal-organic framework for extraction and determination of homo and hetero-polycyclic aromatic hydrocarbons. Talanta 233:122526
Asgharinezhad AA, Rezvani M, Ebrahimzadeh H, Shekari N, Ahmadinasab N, Loni M (2015) Solid phase extraction of Pb (II) and Cd (II) ions based on murexide functionalized magnetic nanoparticles with the aid of experimental design methodology. Anal Methods 7:10350–10358
Asgharinezhad AA, Esmaeilpour M, Siavoshani AY (2022) Extraction and preconcentration of Ni (ii), Pb (ii), and Cd (ii) ions using a nanocomposite of the type Fe3O4@SiO2@polypyrrole-polyaniline. RSC Adv 12:19108–19114
Badruddoza AZM, Shawon ZBZ, Tay WJD, Hidajat K, Uddin MS (2013) Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr Polym 91:322–332
Bagheri H, Molaei K, Asgharinezhad AA, Ebrahimzadeh H, Shamsipur M (2016) Magnetic molecularly imprinted composite for the selective solid-phase extraction of p-aminosalicylic acid followed by high-performance liquid chromatography with ultraviolet detection. J Sep Sci 39:4166–4174
Cao J, Li J, Liu L, Xie A, Li S, Qiu L, Yuan Y, Shen Y (2014) One-pot synthesis of novel Fe3O4/Cu2O/PANI nanocomposites as absorbents in water treatment. J Mater Chem a 2:7953–7959
Comba S, Martin M, Marchisio D, Sethi R, Barberis E (2012) Reduction of nitrate and ammonium adsorption using microscale iron particles and zeolitite. Water Air Soil Pollut 223:1079–1089
Dada AO, Latona DF, Ojediran OJ, Nath OO (2016) Adsorption of cu(II) onto bamboo supported manganese (BS-Mn) nanocomposite: effect of operational parameters, kinetic, isotherms, and thermodynamic studies. J Appl Sci Environ Manag 20:409–422
Dindarloo Inaloo I, Esmaeilpour M, Majnooni S, Reza Oveisi A (2020a) Nickel-catalyzed synthesis of N-(Hetero) aryl carbamates from cyanate salts and phenols activated with cyanuric chloride. ChemCatChem 12:5486–5491
Dindarloo Inaloo I, Majnooni S, Eslahi H, Esmaeilpour M (2020b) Nickel (II) nanoparticles immobilized on EDTA-modified Fe3O4@ SiO2 nanospheres as efficient and recyclable catalysts for ligand-free Suzuki-Miyaura coupling of aryl carbamates and sulfamates. ACS Omega 5:7406–7417
Dindarloo Inaloo I, Majnooni S, Eslahi H, Esmaeilpour M (2020c) Air-Stable Fe3O4@SiO2-EDTA-Ni (0) as an efficient recyclable magnetic nanocatalyst for effective Suzuki-Miyaura and heck cross-coupling via aryl sulfamates and carbamates. Appl Organomet Chem 34:e5662
Duran A, Soylak M, Tuncel SA (2008) Poly (vinyl pyridine-poly ethylene glycol methacrylate-ethylene glycol dimethacrylate) beads for heavy metal removal. J Hazard Mater 155:114–120
Ebrahimzadeh H, Asgharinezhad AA, Tavassoli N, Sadeghi O, Amini MM, Kamarei F (2012) Separation and spectrophotometric determination of very low levels of Cr (VI) in water samples by novel pyridine-functionalized mesoporous silica. Int J Environ Anal Chem 92:509–521
Ebrahimzadeh Mabood H, Khalilzadeh S, Asgharinezhad A, Mehrani Z (2020) Synthesis and application of magnetic ion imprinted polymer nanoparticles for selective extraction and preconcentration of Cd (II) in real samples. Appl Chem 15:135–148
Esmaeilpour M, Javidi J (2015) Fe3O4@ SiO2-imid-PMAn magnetic porous nanosphere as reusable catalyst for synthesis of polysubstituted quinolines under solvent-free conditions. J Chin Chem Soc 62:328–334
Esmaeilpour M, Sardarian AR, Firouzabadi H (2018a) N-heterocyclic carbene-Pd (II) complex based on theophylline supported on Fe3O4@ SiO2 nanoparticles: highly active, durable and magnetically separable catalyst for green Suzuki-Miyaura and Sonogashira-Hagihara coupling reactions. J Organomet Chem 873:22–34
Esmaeilpour M, Sardarian AR, Firouzabadi H (2018b) Theophylline supported on modified silica-coated magnetite nanoparticles as a novel, efficient, reusable catalyst in green one-Pot synthesis of spirooxindoles and phenazines. ChemistrySelect 3:9236–9248
Esmaeilpour M, Zahmatkesh S, Fahimi N, Nosratabadi M (2018c) Palladium nanoparticles immobilized on EDTA-modified Fe3O4@ SiO2 nanospheres as an efficient and magnetically separable catalyst for Suzuki and Sonogashira cross-coupling reactions. Appl Organomet Chem 32:e4302
Esmaeilpour M, Larimi A, Asgharinezhad A, Ghahramanafshar M, Faghihi M (2022) Silica nanoparticles extracted from rice husk and functionalized with dendrimer as an effective recyclable adsorbent to remove divalent cadmium from aqueous solutions. J Appl Polym 6:63–76
Ezati F, Sepehr E, Ahmadi F (2021) The efficiency of nano-TiO2 and γ-Al2O3 in copper removal from aqueous solution by characterization and adsorption study. Sci Rep 11:1–14
Filpponen I, Kontturi E, Nummelin S, Rosilo H, Kolehmainen E, Ikkala O, Laine J (2012) Generic method for modular surface modification of cellulosic materials in aqueous medium by sequential “click” reaction and adsorption. Biomacromol 13:736–742
Fu L, Liu F, Ma Y, Tao X, Ling C, Li A, Shuang C, Li Y (2015) High-efficient technique to simultaneous removal of Cu (II), Ni (II) and tannic acid with magnetic resins: complex mechanism behind integrative application. J Chem Eng 263:83–91
Heydari N, Ghorbani-Kalhor E, Asgharinezhad AA, Bahram M, Vardini MT (2023) Determination of phthalate esters in real matrixes after extraction with a novel magnetic nano-material derived from a metal–organic framework. Microchem J 191:108704
Hsieh SH, Horng JJ (2007) Adsorption behavior of heavy metal ions by carbon nanotubes grown on microsized Al2O3 particles. Int J Miner Metall Mater 14:77–84
Ibrahim M, Siddique A, Verma L, Singh J, Koduru JR (2019) Adsorptive removal of fluoride from aqueous solution by biogenic iron permeated activated carbon derived from sweet lime waste. Acta Chim Slov 66:123–136
Inaloo ID, Majnooni S, Eslahi H, Esmaeilpour M (2020) Efficient nickel (II) immobilized on EDTA-modified Fe3O4@ SiO2 nanospheres as a novel nanocatalyst for amination of heteroaryl carbamates and sulfamates through the cleavage of CO bond. Mol Catal 492:110915
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA, Molaei K (2017) Extraction and determination of trace amounts of gold (III), palladium (II), platinum (II) and silver (I) with the aid of a magnetic nanosorbent made from Fe3O4-decorated and silica-coated graphene oxide modified with a polypyrrole-polythiophene copolymer. Microchim Acta 184:2191–2200
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA (2019) A nanosized magnetic metal-organic framework of type MIL-53 (Fe) as an efficient sorbent for coextraction of phenols and anilines prior to their quantitation by HPLC. Microchim Acta 186:1–8
Jeon Y, Thangadurai DT, Piao L, Yoon S (2013) A facile one-step method to prepare size controlled Fe3O4 submicro/nanoparticles. Mater Lett 96:27–30
Joo S-H, Kim Y-U, Kang J-G, Kumar JR, Yoon H-S, Shin SM (2012) Recovery of rhenium and molybdenum from molybdenite roasting dust leaching solution by ion exchange resins. Mater Trans 53:2034–2037
Karami S, Ebrahimzadeh H, Asgharinezhad AA (2017) A simple and fast method based on functionalized magnetic nanoparticles for the determination of Ag (i), Au (iii) and Pd (ii) in mine stone, road dust and water samples. Anal Methods 9:2873–2882
Kazemnejadi M, Alavi SA, Rezazadeh Z, Nasseri MA, Allahresani A, Esmaeilpour M (2019) Fe3O4@ SiO2@ Im [Cl] Mn (III)-complex as a highly efficient magnetically recoverable nanocatalyst for selective oxidation of alcohol to imine and oxime. J Mol Struct 1186:230–249
Landarani M, Asgharinezhad AA, Ebrahimzadeh H (2020) A magnetic ion-imprinted polymer composed of silica-coated magnetic nanoparticles and polymerized 4-vinyl pyridine and 2, 6-diaminopyridine for selective extraction and determination of lead ions. New J Chem 44:7561–7568
Lu AH, EeL S, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46:1222–1244
Mahdavi S (2016) Nano-TiO2 modified with natural and chemical compounds as efficient adsorbents for the removal of Cd+2, Cu+2, and Ni+2 from water. Clean Techn Environ Policy 18:81–94
Mahima S, Kannikka B, Subhasha N, Moniska J (2018) TiO2-GO nanocomposite for energy and environmental applications: a green syntheis approach. Vacuum 156:434–439
Mahmoud ME, Amira MF, Zaghloul AA, Ibrahim GA (2016) Microwave-enforced sorption of heavy metals from aqueous solutions on the surface of magnetic iron oxide-functionalized-3-aminopropyltriethoxysilane. J Chem Eng 293:200–206
Mangalam J, Kumar M, Sharma M, Joshi M (2019) High adsorptivity and visible light assisted photocatalytic activity of silver/reduced graphene oxide (Ag/rGO) nanocomposite for wastewater treatment. Nano-Struct Nano-Obj 17:58–66
Mashhadizadeh MH, Karami Z (2011) Solid phase extraction of trace amounts of Ag, Cd, Cu, and Zn in environmental samples using magnetic nanoparticles coated by 3-(trimethoxysilyl)-1-propantiol and modified with 2-amino-5-mercapto-1, 3, 4-thiadiazole and their determination by ICP-OES. J Hazard Mater 190:1023–1029
Mehrani Z, Ebrahimzadeh H, Aliakbar AR, Asgharinezhad AA (2018) A poly (4-nitroaniline)/poly (vinyl alcohol) electrospun nanofiber as an efficient nanosorbent for solid phase microextraction of diazinon and chlorpyrifos from water and juice samples. Microchim Acta 185:1–9
Mehrani Z, Ebrahimzadeh H, Asgharinezhad AA, Moradi E (2019) Determination of copper in food and water sources using poly m-phenylenediamine/CNT electrospun nanofiber. Microchem J 149:103975
Mittal H, Maity A, Ray SS (2015) Effective removal of cationic dyes from aqueous solution using gum ghatti-based biodegradable hydrogel. Int J Biol Macromol 79:8–20
Mohapatra RK, Parhi PK, Pandey S, Bindhani BK, Thatoi H, Panda CR (2019) Active and passive biosorption of Pb (II) using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: kinetics and isotherm studies. J Environ Manage 247:121–134
Moradi E, Ebrahimzadeh H, Mehrani Z, Asgharinezhad AA (2019) The efficient removal of methylene blue from water samples using three-dimensional poly (vinyl alcohol)/starch nanofiber membrane as a green nanosorbent. Environ Sci Pollut Res 26:35071–35081
Nassar NN (2010) Rapid removal and recovery of Pb (II) from wastewater by magnetic nanoadsorbents. J Hazard Mater 184:538–546
Panjali Z, Asgharinezhad AA, Ebrahimzadeh H, Karami S, Loni M, Rezvani M, Yarahmadi R, Shahtaheri SJ (2015) Development of a selective sorbent based on a magnetic ion imprinted polymer for the preconcentration and FAAS determination of urinary cadmium. Anal Methods 7:3618–3624
Prabu D, Parthiban R, Senthil Kumar P, Kumari N, Saikia P (2016) Adsorption of copper ions onto nano-scale zero-valent iron impregnated cashew nut shell. Desalin Water Treat 57:6487–6502
Priya AK, Yogeshwaran V, Rajendran S, Hoang TK, Soto-Moscoso M, Ghfar AA, Bathula C (2022) Investigation of mechanism of heavy metals (Cr6+, Pb2+ & Zn2+) adsorption from aqueous medium using rice husk ash: kinetic and thermodynamic approach. Chemosphere 286:131796
Rodriguez-Arco L, Rodriguez IA, Carriel V, Bonhome-Espinosa AB, Campos F, Kuzhir P, Duran JD, Lopez-Lopez MT (2016) Biocompatible magnetic core–shell nanocomposites for engineered magnetic tissues. Nanoscale 8:8138–8150
Sahu N, Nayak AK, Verma L, Bhan C, Singh J, Chaudhary P, Yadav BC (2022) Adsorption of As (III) and As (V) from aqueous solution by magnetic biosorbents derived from chemical carbonization of pea peel waste biomass: Isotherm, kinetic, thermodynamic and breakthrough curve modeling studies. J Environ Manage 312:114948
Sardarian AR, Eslahi H, Esmaeilpour M (2018) Copper (II) complex supported on Fe3O4@ SiO2 coated by polyvinyl alcohol as reusable nanocatalyst in N-arylation of amines and N (H)-heterocycles and green synthesis of 1H-tetrazoles. Chem Select 3:1499–1511
Sardarian AR, Eslahi H, Esmaeilpour M (2019) Green, cost-effective and efficient procedure for Heck and Sonogashira coupling reactions using palladium nanoparticles supported on functionalized Fe3O4@ SiO2 by polyvinyl alcohol as a highly active, durable and reusable catalyst. Appl Organomet Chem 33:e4856
Sardarian A, Kazemnejadi M, Esmaeilpour M (2021) Functionalization of superparamagnetic Fe3O4@ SiO2 nanoparticles with a Cu (II) binuclear Schiff base complex as an efficient and reusable nanomagnetic catalyst for N-arylation of α-amino acids and nitrogen-containing heterocycles with aryl halides. Appl Organomet Chem 35:e6051
Sharma RK, Agrawal M (2005) Biological effects of heavy metals: an overview. J Environ Biol 26:301–313
Sharma M, Joshi M, Nigam S, Shree S, Avasthi DK, Adelung R, Srivastava SK, Mishra YK (2019) ZnO tetrapods and activated carbon based hybrid composite: Adsorbents for enhanced decontamination of hexavalent chromium from aqueous solution. J Chem Eng 358:540–551
Soleimani M, Afshar MG (2013) Potentiometric sensor for trace level analysis of copper based on carbon paste electrode modified with multi-walled carbon nanotubes. Int J Electrochem Sci 8:8719–8729
Soleimani M, Afshar MG (2014) Octaethylporphyrin as an ionophore for aluminum potentiometric sensor based on carbon paste electrode. Russ J Electrochem 50:554–560
Soleimani M, Afshar MG (2015) Highly selective solid phase extraction of mercury ion based on novel ion imprinted polymer and its application to water and fish samples. J Anal Chem 70:5–12
Soleimani M, Mahmodi MS, Morsali A, Khani A, Afshar MG (2011) Using a new ligand for solid phase extraction of mercury. J Hazard Mater 189:371–376
Soleimani M, Ghahraman Afshar M, Sedghi A (2013) Amino-functionalization of multiwall carbon nanotubes and its use for solid phase extraction of mercury ions from fish sample. Int Scholarly Res Notices. https://doi.org/10.1155/2013/674289
Somaratne MC, Liyanage NM, Walpalage S. (2014) Surface modification of silica with a hydrophilic polymer and its influence on reinforcement of natural rubber latex. Journal of the National Science Foundation of Sri Lanka. 42(4).
Soni S, Bajpai P, Mittal J, Arora C (2020) Utilisation of cobalt doped Iron based MOF for enhanced removal and recovery of methylene blue dye from waste water. J Mol Liq 314:113642
Tadjarodi A, Abbaszadeh A, Taghizadeh M, Shekari N, Asgharinezhad AA (2015) Solid phase extraction of Cd (II) and Pb (II) ions based on a novel functionalized Fe3O4@ SiO2 core-shell nanoparticles with the aid of multivariate optimization methodology. Mater Sci Eng C 49:416–421
Tripathi PK, Liu M, Zhao Y, Ma X, Gan L, Noonan O, Yu C (2014) Enlargement of uniform micropores in hierarchically ordered micro–mesoporous carbon for high level decontamination of bisphenol A. J Mater Chem a 2:8534–8544
Venkateswarlu S, Rao YS, Balaji T, Prathima B, Jyothi N (2013) Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract. Mater Lett 100:241–244
Verma L, Singh J (2019) Synthesis of novel biochar from waste plant litter biomass for the removal of Arsenic (III and V) from aqueous solution: a mechanism characterization, kinetics and thermodynamics. J Environ Manage 248:109235
Verma L, Singh J (2023) Arsenic adsorption from aqueous solution and groundwater using monometallic (Fe) and bimetallic (Fe/Mn) Tectona biochar synthesized from plant refuse: mechanism, isotherm, and kinetic study. Environ Eng Res 28:220110–220123
Verma L, Siddique MA, Singh J, Bharagava RN (2019) As (III) and As (V) removal by using iron impregnated biosorbents derived from waste biomass of Citrus limmeta (peel and pulp) from the aqueous solution and ground water. J Environ Manage 250:109452
Verma L, Azad A, Singh J (2022) Performance of a novel iron infused biochar developed from Raphanus sativus and Artocarpus heterophyllus refuse for trivalent and pentavalent arsenic adsorption from an aqueous solution: mechanism, isotherm and kinetics study. Int J Phytoremed 24:919–932
Xiao J, Hu R, Chen G, Xing B (2020) Facile synthesis of multifunctional bone biochar composites decorated with Fe/Mn oxide micro-nanoparticles: physicochemical properties, heavy metals sorption behavior and mechanism. J Hazard Mater 399:123067
Xiong Y, Ye F, Zhang C, Shen S, Su L, Zhao S (2015) Synthesis of magnetic porous γ-Fe2O3/C@ HKUST-1 composites for efficient removal of dyes and heavy metal ions from aqueous solution. RSC Adv 5:5164–5172
Xu M, Zhang Y, Zhang Z, Shen Y, Zhao M, Pan G (2011) Study on the adsorption of Ca2+, Cd2+ and Pb2+ by magnetic Fe3O4 yeast treated with EDTA dianhydride. Chem Eng J 168:737–745
Yaneva ZL, Koumanova BK, Allen SJ (2013) Applicability comparison of different kinetic/diffusion models for 4-nitrophenol sorption on Rhizopus oryzae dead biomass. Bulg Chem Commun 45:161–168
Yuan D, Anthis AH, Ghahraman Afshar M, Pankratova N, Cuartero M, Crespo GA, Bakker E (2015) All-solid-state potentiometric sensors with a multiwalled carbon nanotube inner transducing layer for anion detection in environmental samples. Anal Chem 87:8640–8645
Zahmatkesh S, Esmaeilpour M, Javidi J (2016) 1, 4-Dihydroxyanthraquinone–copper (II) supported on superparamagnetic Fe3O4@ SiO2: An efficient catalyst for N-arylation of nitrogen heterocycles and alkylamines with aryl halides and click synthesis of 1-aryl-1, 2, 3-triazole derivatives. RSC Adv 6:90154–90164
Zhang H, Zhong X, Xu J-J, Chen H-Y (2008) Fe3O4/polypyrrole/Au nanocomposites with core/shell/shell structure: synthesis, characterization, and their electrochemical properties. Langmuir 24:13748–13752
Zhang J, Ma X, Yuan L, Zhou D (2020) Comparison of adsorption behavior studies of Cd2+ by vermicompost biochar and KMnO4-modified vermicompost biochar. J Environ Manag 256:109959
Zhong L-S, Hu J-S, Cao A-M, Liu Q, Song W-G, Wan L-J (2007) 3D flowerlike ceria micro/nanocomposite structure and its application for water treatment and CO removal. Chem Mater 19:1648–1655
Acknowledgements
The authors thank the Niroo Research Institute (NRI) and the Estahban Payam Noor University for supporting this research.
Funding
Niroo Research Institute (NRI) supports the project fanatically.
Author information
Authors and Affiliations
Contributions
AAA contributed to conceptualization, software, formal analysis, data curation, writing-original draft preparation, reviewing and editing, visualization, investigation. ME contributed to supervision, conceptualization, methodology, formal analysis, data curation, writing—reviewing and editing, visualization, investigation. MGA contributed to conceptualization, methodology, formal analysis, validation, investigation, reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
There is not any competing interest in all manuscript.
Ethical approval
There is not any experiment which involves human precipitants.
Consent to participate
All authors are informed about the submission of this research.
Consent for publication
The authors give the permission to the publisher to publish this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asgharinezhad, A.A., Esmaeilpour, M. & Afshar, M.G. Synthesis of magnetic Fe3O4@SiO2 nanoparticles decorated with polyvinyl alcohol for Cu(II) and Cd(II) ions removal from aqueous solution. Chem. Pap. 78, 3799–3814 (2024). https://doi.org/10.1007/s11696-024-03350-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-024-03350-4