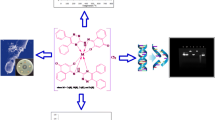
Abstract
In the current work, non-toxic and safe Ni(II) complexes of unsymmetrical Schiff base ligand derived from Quinoline hybrid and isoniazid, viz. [NiII(Quibal-INH)2] (1), [NiII(Quibal-INH)(cat)] (2) and [NiII(Quibal-INH)(bha)] (3), were synthesized. The complexes were characterized by elemental analysis and ATR-IR spectroscopy. These novel complexes were further preliminary evaluated for their solution stability in varied pH conditions. Furthermore, the interactions between Ni (II) complexes with human serum albumin (HSA) have been explored by using UV–visible spectroscopy. It was inferred that the complexes could quench the intrinsic fluorescence of HSA in a steady quenching process. The compounds were tested for their therapeutic potentials, viz. antibacterial, antifungal and anti-TB potential. In preliminary screening, nickel complexes (1) and (3) were active against Candida albicans with excellent growth inhibition. Nickel complex (1) was established as active and was assigned for further validation. For complexes 1 and 3, a toxicity test was performed on human embryonic kidney cells, and a hemolytic test was conducted on human red blood cells. Both complexes (1 and 3) were found to be non-toxic and safe. Nickel Complex (2) exhibited excellent anti-TB activity against the MTB H37Rv strain, which was comparable to the standard TB drugs.
Graphical abstract







Similar content being viewed by others
References
Ahmed AH, Gumaa HA, Mohamed BH, Eraky AM (2016) Nickel(II)-oxaloyldihydrazone complexes: characterization, indirect band gap energy and antimicrobial evaluation. Cogent Chem 2:1142820
Bottari B, Maccari R, Monforte F, Ottanà R, Rotondo E, Vigorita MG (2000) Isoniazid-related copper(II) and nickel(II) complexes with antimycobacterial in vitro activity. Part 9. Bioorg Med Chem Lett 10:657–660
Cascioferro S, Barbara P, Daniela C, Domenico S, Elisa G, Girolamo C, Patrizia D (2020) Thiazoles, their benzofused systems, and thiazolidinone derivatives: versatile and promising tools to combat antibiotic resistance. J Med Chem 63:7923–7956
Correia I, Adao P, Roy S, Wahba M, Matos C, Maurya MR et al (2014) Hydroxyquinoline derived vanadium (IV and V) and copper (II) complexes as potential anti-tuberculosis and anti-tumor agents. J Inorg Biochem 141:83–93
Das K, Sanwlani S, Rawat K, Haughn CR, Doty MF, Bohidar H (2016) Spectroscopic profile of surfactant functionalized CdSe quantum dots and their interaction with globular plasma protein BSA. Coll Surf A Physicochem Eng Asp 506:495–506
de Kraker ME, Stewardson AJ, Harbarth S (2016) Will 10 million people die a year due to antimicrobial resistance by 2050. PLoS Med 13:e1002184
Dinakaran M, Senthilkumar P, Yogeeswari P, China A, Nagaraja V, Sriram D (2008) Antimycobacterial activities of novel 2-(sub)-3-fluoro/nitro-5,12-dihydro-5-oxobenzothiazolo[3,2-a]quinoline-6-carboxylic acid. Bioorg Med Chem 16:3408–3418
Fleck M, Layek M, Saha R, Bandyopadhyay D (2013) Synthetic aspects, crystal structures and antibacterial activities of manganese(III) and cobalt(III) complexes containing a tetradentate Schiff base. Transit Metal Chem 38:715–724
Gerlier D, Thomasset N (1986) Use of MTT colorimetric assay to measure cell activation. J Imm Methods 94:57–63
Hegde GS, Bhat SS, Netalkar SP, Hegde PL, Kotian A, Butcher RJ, Revankar VK (2021) The Co(II), Ni(II), Cu(II) and Zn(II) complexes of aroylhydrazone of quinolone core: syntheses, characterization and evaluation of antimicrobial and antitubercular activity. Inorg Chim Acta 522:120352
Islam MR, Islam SM, Noman AS, Khanam JA, Ali SM, Alam S, Lee MW (2007) Biological screening of a novel nickel (II) tyrosine complex. Mycobiology 35:25–29
Jash C, Kumar GS (2014) Binding of alkaloids berberine, palmatine and coralyne to lysozyme: a combined structural and thermodynamic study. RSC Adv 4:12514–12525
Jayaprakash S, Iso Y, Wan B, Franzblau SG, Kozikowski AP (2006) Design, synthesis, and SAR studies of mefloquine-based ligands as potential antituberculosis agents. Chem Med Chem 1:593–597
Kongot M, Dohare N, Singh V, Reddy DS, Singhal NK, Patel R, Kumar A (2018) A novel biocompatible Ni II tethered moiety as a glucose uptake agent and a hit against methicillin-resistant Staphylococcus aureus. Eur J Pharm Sc 123:335–349
Kongot M, Reddy D, Singh V, Patel R, Singhal NK, Kumar A (2019a) Potent drug candidature of an ONS donor tethered copper (II) complex: anticancer activity, cytotoxicity and spectroscopically approached BSA binding studies. Spectrochim Acta A Mol Biomol Spectrosc 212:330–342
Kongot M, Dohare N, Reddy DS, Pereira N, Patel R, Subramanian M, Kumar A (2019b) In vitro apoptosis-induction, antiproliferative and BSA binding studies of a oxidovanadium (V) complex. J Trace Elem Med Bio 51:176–190
Lilienkampf A, Mao J, Wan B, Wang Y, Franzblau SG, Kozikowski AP (2009) Structure-activity relationships for a series of quinoline-based compounds active against replicating and nonreplicating Mycobacterium tuberculosis. J Med Chem 52:2109–2118
Liu Z-C, Wang B-D, Yang Z-Y, Li Y, Qin D-D, Li T-R (2009) Synthesis, crystal structure, DNA interaction and antioxidant activities of two novel water-soluble Cu2+ complexes derivated from 2-oxo-quinoline-3-carbaldehyde Schiff-bases. Eur J Med Chem 44:4477–4484
Lourenco MC, de Souza MV, Pinheiro AC, Ferreira MDL, Gonçalves RS, Nogueira TCM, Peralta MA (2007) Evaluation of anti-tubercular activity of nicotinic and isoniazid analogues. ARKIVOC 15:181–191
Mandewale MC, Patil UC, Shedge SV, Dappadwad UR, Yamgar RS (2017) A review on quinnline hydrazone derivatives as a new class of potent antitubercular and anticancer agents. Beni-Suef Univ J Basic Appl Sci 6:354–361
Mandewale MC, Thorat B, Nivid Y, Jadhav R, Nagarsekar A, Yamgar R (2018) Synthesis, structural studies and antituberculosis evaluation of new hydrazone derivatives of quinoline and their Zn(II) complexes. J Saudi Chem Soc 22:218–228
Musiol R, Jampilek J, Buchta V, Silva L, Niedbala H, Podeszwa B, Palka A, Majerz-Maniecka K, Oleksyn B, Polanski J (2006) Antifungal properties of new series of quinoline derivatives. Bioorg Med Chem 14:3592–3598
Nayyar A, Monga V, Malde A, Coutinho E, Jain R (2007) Synthesis, anti-tuberculosis activity, and 3D-QSAR study of 4-(adamantan-1-yl)-2-substituted quinolines. Bioorg Med Chem 15:626–640
Pan X, Liu R, Qin P, Wang L, Zhao X (2010) Spectroscopic studies on the interaction of acid yellow with bovine serum albumin. J Lumin 130:611–617
Raj P, Singh A, Singh A, Singh N (2017) Syntheses and photophysical properties of schiff base Ni(II) complexes: application for sustainable antibacterial activity and cytotoxicity. ACS Sustain Chem Eng 5:6070–6080
Reddy DS, Kongot M, Netalkar SP, Kurjogi MM, Kumar R, Avecilla F, Kumar A (2018) Synthesis and evaluation of novel coumarin-oxime ethers as potential anti-tubercular agents: their DNA cleavage ability and BSA interaction study. Eur J Med Chem 150:864–875
Reddy DS, Kongot M, Singh V, Maurya N, Patel R, Singhal NK, Avecilla F, Kumar A (2019) Coumarin tethered cyclic imides as efficacious glucose uptake agents and investigation of hit candidate to probe its binding mechanism with human serum albumin. Bioorg Chem 92:103212
Reddy DS, Kongot M, Kumar A (2021a) Coumarin hybrid derivatives as promising leads to treat tuberculosis: recent developments and critical aspects of structural design to exhibit anti-tubercular activity. Tuberculosis 127:102050
Reddy DS, Kongot M, Singh V, Siddiquee MA, Patel R, Singhal NK, Avecilla F, Kumar A (2021b) Biscoumarin-pyrimidine conjugates as potent anticancer agents and binding mechanism of hit candidate with human serum albumin. Arch Pharm (weinheim) 354:e2000181
Reddy DS, Sinha A, Kumar A, Saini VK (2022) Drug re-engineering and repurposing: a significant and rapid approach to tuberculosis drug discovery. Arch Pharm 355:2200214
Regiel-Futyra A, Dąbrowski JM, Mazuryk O, Śpiewak K, Kyzioł A, Pucelik B, Brindell M, Stochel G (2017) Bioinorganic antimicrobial strategies in the resistance era. C Chem Rev 351:76–117
Sain S, Saha R, Mostafa G, Fleck M, Bandyopadhyay D (2012) Synthesis and crystal structure of three new copper (II) complexes with a tridentate amine and its Schiff bases. Polyhedron 31:82–88
Savini L, Chiasserini L, Gaeta A, Pellerano C (2002) Synthesis and anti-tubercular evaluation of 4-quinolylhydrazones. Bioorg Med Chem 10:2193–2198
Singh K, Kumar Y, Puri P, Sharma C, Aneja KR (2017) Antimicrobial, spectral and thermal studies of divalent cobalt, nickel, copper and zinc complexes with triazole Schiff bases. Arab J Chem 10:978–987
Sinha A, Chaudhary R, Reddy DS, Kongot M, Kurjogi MM, Kumar A (2022) ON donor tethered copper (II) and vanadium (V) complexes as efficacious anti-TB and anti-fungal agents with spectroscopic approached HSA interactions. Heliyon 8:e10125
Sridhar G, Bilal IM, Easwaramoorthy D, Rani SK, Kumar BS, Sai MC (2017) Synthesis, characterization and antimicrobial activities of copper, nickel, cobalt, chromium complexes derived from (Z)-4-Fluoro-N-(2,7-dimethylhept-6-enylidene) benzenamine. J Braz Chem Soc 28:756–767
Tabong CD, Yufanyi DM, Paboudam AG, Nono KN, Eni DB, Agwara MO (2016) Synthesis, crystal structure, and antimicrobial properties of [diaquabis (hexamethylenetetramine) diisothiocyanato-κN] nickel (II) complex. Adv Chem 2016:1–8
Udwadia ZF, Vora A, Tripathi AR, Malu KN, Lange C, Raju RS (2020) COVID-19-Tuberculosis interactions: when dark forces collide. Ind J Tuberc 67:S155–S162
Yano S, Inoue S, Nouchi R, Mogami K, Shinohara Y, Yasuda Y, Kato M, Tanase T, Kakuchi T, Mikata Y, Suzuki T, Yamamoto Y (1998) Antifungal nickel(II) complexes derived from amino sugars against pathogenic yeast. Candida AlbicansJ Inorg Biochem 69:15–23
World Health Organization, Antimicrobial resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance Accessed Oct (2022).
B Erickson (2020) Extent of antibiotic resistance unknown. C & EN. Vol 98, https://cen.acs.org/pharmaceuticals/antibiotics/Extent-antibiotic-resistance-unknown-report/98/i18
MTT Cell proliferation assay instruction guide ATCC, VA, USA (www.atcc.org) Accessed Nov 2022
World Health Organization, Global Tuberculosis Report (2019) https://www.who.int/tb/global-report-2019Accessed Jan 2023.
The potential impact of the COVID-19 response on Tuberclosis in high-burden countries: a modelling analysis https://stoptb.org/assets/documents/news/Modeling%20Report_1%20May%202020_FINAL.pdf Accessed Dec 2022.
K Todar (2011) Bacterial mechanisms of antibiotic resistance. Todar’s online textbook of bacteriology, Scientific Research, http://textbookofbacteriology.net/resantimicrobial_3.html
Acknowledgements
CNMS, Jain University, Bangalore, India, provided funding to Prof. Amit Kumar and Dr. Dinesh Reddy to carry out this research work, with reference numbers JU/MRP/CNMS/2/2022 and JU/MRP/CNMS/8/2022 respectively.
Author information
Authors and Affiliations
Contributions
MK contributed to conceptualization, data curation, investigation, methodology, resources, validation, visualization, writing—original draft, writing—review and editing. RC contributed to methodology, validation, visualization, resources, and writing—review and editing. DSR contributed to methodology, validation, visualization, resources, writing—review and editing. Anamika Sinha contributed to validation, visualization, resources, writing—review and editing. MMK contributed to validation, resources, writing—review and editing. AK contributed to project administration, funding acquisition, investigation, resources, visualization, and writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kongot, M., Chaudhary, R., Reddy, D.S. et al. Synthesis, characterization, antimicrobial and antitubercular evaluation of nickel (II) complexes of ON donor quinoline-hydrazide core. Chem. Pap. 78, 3223–3232 (2024). https://doi.org/10.1007/s11696-024-03307-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-024-03307-7