Abstract
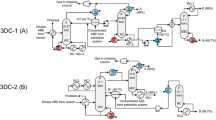
This work is a study of the design of a process for the separation of aqueous mixtures containing ethanol (Etha) and acetic acid (AcA) by distillation processes. The process developed for the separation of three cases of high water (W) composition mixtures was simulated by the Aspen Plus V10 software. They regressed the binary interaction parameters of the NRTL model from the existing experimental results. The process comprises three successive columns, the first distillation column with 36 stages where the acetic acid is recovered at the bottom of the column at 99.9% molar. The distillate stream got at the top of this column feeds the second extractive distillation column with 32 stages in the presence of ethylene glycol (Ethy) to separate ethanol with a purity higher than 99.20% mol. At the top of this extractive distillation column and then the bottom stream of this extractive distillation column feeds the third regeneration column with 18 stages to recover ethylene glycol at the bottom with a purity of 99.99% molar which is recycled to the second column with a make-up of 0.37%. Side draw stream is added to stage 4 of the first distillation column. The side draw allows a reduction in energy consumption of 22% compared to a distillation process without a side draw for a feed containing 10% molar acetic acid, 35% molar ethanol, and 55% molar water.












Similar content being viewed by others
Abbreviations
- A ij, B ij, C ij :
-
The binary parameters for the NRTL model.
- AcA:
-
Acetic acid
- Ap:
-
Appoint
- Ethy:
-
Ethylene glycol
- Etha:
-
Ethanol
- G ij :
-
Gibbs energy
- MRI:
-
Mean relative uncertainty
- MW:
-
Mega watts
- P:
-
Pressure
- R:
-
Recycle
- T exp :
-
Experimental temperature
- T cal :
-
Calculated temperature
- RMS:
-
Root-mean-square
- x:
-
Mole fraction in liquid vapor
- y exp :
-
Experimental mole fraction in the vapor phase
- y cal :
-
Calculated mole fraction in the vapor phase
- W:
-
Water
- τ ji :
-
Binary interaction parameter between i and j
- γ i :
-
Activity coefficient of compound i
- α ij :
-
NRTL non-random factor
References
Alriols MG, Tejado A, Blanco M, Mondragon I, Labidi, (2009) Agricultural palm oil tree residues as raw material for cellulose, lignin and hemicelluloses production by ethylene glycol pulping process. Chem Eng J. https://doi.org/10.1016/j.cej.2008.08.008
Beneke D, Peters D (2012) Understanding Distillation Using Column, Glasser and Hildebrandt, ed. Wiley. ISBN: 978–1–118–47729–8
Bowen TC, Vane LM (2006) Ethanol, acetic acid, and water adsorption from binary and ternary liquid mixtures on high-silica zeolites. Langmuir 22(8):3721–3727. https://doi.org/10.1021/la052538u
Calvar N, González B, Dominguez A (2007) Esterification of acetic acid with ethanol: reaction kinetics and operation in a packed bed reactive distillation column. Chem Eng Process: Process Intensification 46(12):1317–1323. https://doi.org/10.1016/j.cep.2006.10.007
Chen G, Wang Q, Zhang L, Bao J, Han S (1995) Study and applications of binary and ternary azeotropes. Thermochim Acta 253:295–305
Cousin Saint Remi J, Baron G, Denayer J (2012) Adsorptive separations for the recovery and purification of biobutanol. Adsorption 18:367–437
Doherty MF, Malone MF (2001) Conceptual Design of Distillation Systems. Mc Graw Hill, New York
Drapcho C, Nghiem N, Walker T (2020) Ethanol Production. In Biofuels Engineering Process Technology, 2nd edn. McGraw-Hill, pp 117–271
Efremova SA, Komarova LF, Garber YuI, Tikhanovich EV (1988) Phase equilibria in binary components of the Acetone-ethanol-water-methyl cellosolve-ethylene glycol system. J Appl Chem USSR 61:2359–2361
ÉTHYLÈNE GLYCOL (2000) Environnement Canada, Santé Canada, Loi canadienne sur la protection de l’environnement.
Gerbaud V, Rodriguez D I (2014) Extractive distillation. In: Gorak A, Olujic Z. (Eds.), Distillation Book, Vol. II Distillation: Equipment and Processes. Elsevier, Amsterdam, pp.201–246, ISBN 978–0–12–386878–7, Chapter 6.
Global Ethanol Production by Country or Region. https://afdc.energy.gov/data/10331/ Accessed on 19 September 2021.
Graves T, Narendranath NV, Dawson Karl, Power Ronan (2006) Effect of pH and lactic or acetic acid on ethanol productivity by Saccharomyces cerevisiae in corn mash. J Ind Microbiol Biotechnol. https://doi.org/10.1007/s10295-006-0091-6
Halder P, Azad K, Shah S, Sarker E (2019) Prospects and technological advancement of cellulosic bioethanol ecofuel production. In: Advances in Eco-Fuels for a Sustainable Environment, pp 211–236. https://doi.org/10.1016/B978-0-08-102728-8.00008-5
Ikegami T, Yanagishita H, Kitamoto D, Haraya K, Nakane T, Matsuda H, Koura N, Sano T (1999) Highly concentrated aqueous ethanol solutions by pervaporation using silicalite membrane — Improvement of ethanol selectivity by addition of sugars to ethanol solution. Biotechnol Lett. https://doi.org/10.1023/a:1005627421221
Ito T, Yoshida F (1963) Vapor-liquid equilibria of water-lower fatty acid systems. water- formic acid, watrer-acetic acid and water-propionic acid. J Chem Eng Data 8:315–320
Iwakabe K, Kosuge H (2001) Isobaric vapor-liquid-liquid equilibiria with a newly developed still. Fluid Phase Equilib 192:171–186
Kamihama N, Matsuda H, Kurihara K, Tochigi K, Oba SJ (2012) Isobaric vapor-liquid equilibria for ethanol + water + ethylene glycol and Its constituent three binary systems. Chem Eng Data 57(2):339–344. https://doi.org/10.1021/je2008704
Lai Hung-Sheng, Lin Yi-Feng, Chein-Hsiun Tu (2014) Isobaric (vapor+liquid) equilibria for the ternary system of (ethanol+water+1,3-propanediol) and three constituent binary systems at P=101.3kPa. J Chem Thermodyna 68:13–19. https://doi.org/10.1016/j.jct.2013.08.020
Lang P, (1992) Computation of multi stage multi component separation processes. In: Pallai, I., Fonyo, Z.s. (Eds.), Studies in Computer-aided Modelling, Design and Operation: Unit Operations Pt. A Operations. Elsevier, London, pp. 256–399, ISBN:978–0444986733. Chapter 7.
Liang S, Cao Y, Liu X, Li X, Zhao Y, Wang Y, Wang Y (2017) Insight into pressure-swing distillation from azeotropic phenomenon to dynamic control. Chem Eng Res Des. https://doi.org/10.1016/j.cherd.2016.10.040
Luyben WL (2005) Control of a multiunit heterogeneous azeotropic distillation process. Wiley Inter Sci. https://doi.org/10.1002/aic.10650
Luyben WL, Chien IL (2010) Design and Control of Distillation Systems for Separating Azeotropes. Wiley-VCH, NewYork, p 453p
Mathias P (2017) Guidelines for the analysis of vapor−liquid equilibrium data. J. Chem. Eng. Data 62:2231–2233. https://doi.org/10.1021/acs.jced.7b00582
Mujtaba IM (1999) Optimization of batch extractive distillation processes for separating close boiling and azeotropic mixtures. Chem Eng Res Des 77:588–596
Peng Y, Liang J, Ping L, Mao J, Zhu H (2009) Thermodynamic consistency for the vapor liquid equilibrium data of associating system. Acta Petrolei Sinica, 25(5), 8; 717–724. http://www.syxbsyjg.com/EN/Y2009/V25/I5/717
Petlyuk F B, (2004) Distillation Theory and Its Application to Optimal Design of Separation Units. Cambridge Series in Chemical Engineering, Cambridge University Press, Cambridge, UK.
Renon H, Prausnitz JM (1968) Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J 14:135–144. https://doi.org/10.1002/aic.690140124
Schmid B, Döker M, Gmehling J (2008) Esterification of ethylene glycol with acetic acid catalyzed by amberlyst 36. Ind Eng Chem Res. https://doi.org/10.1021/ie0707117
Segovia HJG, Hernández S, Bonilla Petriciolet A (2015) Reactive distillation: a review of optimal design using deterministic and stochastic techniques. Chem Eng Res Des. https://doi.org/10.1016/j.cep.2015.09.004
Stichlmair JG, Fair JR (1998) Distillation: Principles and Practices. Wiley, NewYork
Tengborg C, Galbe M, Zacchi G (2001) Reduced inhibition of enzymatic hydrolysis of steam-pretreated softwood. Enzyme Microb Technol. https://doi.org/10.1016/S0141-0229(01)00342-8
Trinh L T P, Lee J, Lee H (2016) Acidified glycerol pretreatment for enhanced ethanol production from rice straw. Biomass Bioenerg.
Tripathy SS, Raichur AM (2008) Enhanced adsorption capacity of activated alumina by impregnation with alum for removal of As(V) from water. Chemical Engineering Journal 138(1–3):179–186. https://doi.org/10.1016/j.cej.2007.06.028
Udeye V, Mopoung S, Vorasingha A, Amornsakcha P (2009) Ethanol heterogeneous azeotropic distillation design and construction. Int J Phys Sci 4 (3): 101–106. ISSN 1992 - 1950 © 2009
Wang H, Yang B, Huaxue Gongcheng (2002) Measurement of Isobaric Vapor-Liquid Equilibrium Data for the System of Ether-Alcohol-Water. Huaxue Gongcheng 30(2), 8;57
Zhu D, Gao D, Zhang H, Winter B, Lucking P, Sun H, Guan H, Chen H, Shi JJ (2013) Chem Eng Data 58(1):41–47
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahida, B., Medjahdi, M., Khelifa, I. et al. Separation of acetic acid and ethanol from wastewater by distillation process with addition of side draw stream. Chem. Pap. 77, 837–846 (2023). https://doi.org/10.1007/s11696-022-02516-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02516-2