Abstract
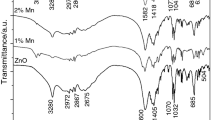
The ZnAlO and Mn (Manganese), Dy (Dysprosium) doped ZnAlO compounds i.e., ZnAlOMn and ZnAlODy compound powder synthesized by Sol–Gel Technique (SGT). Lattice Strain (LS) produced by Ball Milling Technique (BMT), by repeating grinding/milling produces LS and it has decreased Particle Size (PS). Debye temperature (DT) is represented by θM, Debye–Waller Factor (DWF) is represented by B and Amplitude of Vibrations (AV) is represented by < u2 > . The values of LS, PS, DT, DWF and AV of ZnAlO, Mn-doped ZnAlO, Dy doped ZnAlO compounds were calculated by using X-Ray Diffraction (XRD) method. The powder samples were equipped/prepared with Zinc acetate (ZnC6H6O4), aluminum acetate (C6H9AlO6), Manganese acetate (C4H6MnO4), dysprosium acetate (C6H12DyO6) respectively. 1 M of ZnC4H6O4, 1 M of C6H9AlO6 was added/dissolved in 2 methoxy ethanol (C3H8O2). Acidic acid (CH3COOH) and Ethylene glycol (C2H6O2) were added 1:1 ratio to the ancestor/precursor solution. Ammonium hydroxide (NH4OH) added to the consequential solution to adjust the PH value. The solution determination keep under constant stirring at 80 °C temperature using magnetic stirrer and dried at 80 °C temperature. Final sol–gel resultant product was annealed at 900 °C for 2 h. The Mn and Dy were also doped with ZnAl2O4 and synthesized in the above-mentioned same procedure have been used. The SGT Resultant powder has been milling 2, 4 h using the ball mill. The XRD patterns were recorded for ZnAlO, ZnAlOMn and ZnAlODy powders and also BMT resultant powders. The DT, DWF and AV were obtained from integrated intensities of X-rays. The LS and PS have been calculated by using Williamson-Hall Technique (WHT). In this work, Vacancy Formation Energy (VFE) has been also evaluated.


Similar content being viewed by others
References
Bharati R, Rehani PB, Joshi KN (2006) Crystallite size estimation of elemental and composite silver nano-powders using XRD principles. Lad and Arun Pratap. Indian J Pure Appl Phys 44:157–161
Chipman DR, Paskin A (1959) Temperature dependence of the Debye temperatures of aluminum, lead, and beta brass by an x-ray method. J Appl Phys 30:1938
Glyde HR (1967) Relation of vacancy formation and migration energies to the Debye temperature in solids. J Phys Chem Solids 28:2061
Gopi Krishna N, Sirdeshmukh DB (1988) Compilation of temperature factors of hexagonal close packed elements. Acta Crystallography A 54:513–514
Gopi Krishna N, Sirdeshmukh DB (1993) X-ray studies of lattice strain and Debye-Waller factors of ytterbium. Indian J Pure Appl Phys 31:198
Gopi Krishna N, Sirdeshmukh DB, Rama Rao B, Beandry BJ, Jr.Gschneidner KA (1986) Mean square amplitudes of vibration and associated Debye temperatures of dysprosium, gadolinium, lutetium and yttrium. Indian J Pure Appl Phys 24:324–326
Gopi Krishna N, Sirdeshmukh DB, Gscheneidner Jr KA (1988) X-ray determination of mean square amplitudes of vibration and associated Debye temperatures of scandium and terbium. Indian J Pure Appl Phys 26:724–728
Gopi Krishna N et al (2010) Effect of lattice strain on the Debye-Waller factors of Mg. Zn Cd Indian J Phys 84(7):887
Inagaki M, Furuhashi H, Ozeki T et al (1971) Heat treatment of mesocarbon microbeads under high pressure. J Mater Sci 6:1520
Inagaki M, Furuhashi H, Ozeki T, Naka S (1973) Integrated intensity changes for crystalline powders by grinding and compression changes in effective temperature factor. J Mater Sci 8:312
International tables for X ray crystallography, Vol III. Kynoch press, Birmingham (1968).
James RW (1967) The optical principles of the diffraction of X-Rays. Cornel University Press, Ithaca
Jithender L, Karunakara M (2019) Debye temperature study of some metal compound powders. AIP Conf Proc 2104:020016. https://doi.org/10.1063/1.5100384
Kaelble EF (1967) Handbook of X-rays. Mc Grawhill, New York
Klein L (1994) Sol-Gel Optics: processing and applications. In: Lisa C. Klein (ed) Kluwer. Sol-Gel Optics: Processing and Applications. Springer, Cham, 592, ISBN: 0-7923-9424-0
Micro-and macro-properties of solids (2006) Springer Series in Material Science, Cham.
Purushotham E (2014b) X-Ray study of strined metal powders. J Adv Phys 3(1):55–59. https://doi.org/10.1166/jap.2014.1093
Purushotham E (2014c) Compilation and anisotropy of DebyeWaller factors of hexagonal close packed elements. J Adv Phys 3(1):60–63. https://doi.org/10.1166/jap.2014.1095
Purushotham E (2014d) Mean square amplitudes of vibration and associated Debye temperatures of Be, Sc and Ru. J Adv Phys 3(1):67–69. https://doi.org/10.1166/jap.2014.1094
Purushotham E (2013) Effect of particle size and lattice strain on the debyewaller factors of copper (Cu) powder using high 4haract ball mill. J Eng Sci Technol Rev 6(1):83–86.
Purushotham E (2014a) Effect of particle size and lattice strain on the DebyeWaller factors of Fe3C Nanoparticles. Bull Mater Sci 37(4):773–778
Purushotham E (2016a) Preparation and characterization of metal carbide nanoparticles. Chem Mater Res 8(3):23–28.
Purushotham E (2016b) Effect of Lattice strain on the Debye-Waller factor of CuInSe2 Nanoparticles. Am J Mater Syn Process 1(4):43–46. https://doi.org/10.11648/j.ajmsp.2016b0104.12
Purushotham E (2016c) Effect of particle size and lattice strain on the Debyewaller factors of silicon carbide nanoparticles. J Nanosci Nanotechnol (SCI Journal) 5(3): 2658–2662. https://doi.org/10.1166/jnn.2016.12462
Purushotham E (2018) Effect of vacancy formation energy and microhardness on the Debye temperatures of some α-Phase alloys. Int J High Energy Phys 5(1):1–4. https://doi.org/10.11648/j.ijhep.20180501.11
Purushotham E (2019a) Preparation and characterization of CuInSe2 nano-particles. Rasayan J Chem 12(4):1676–1680. https://doi.org/10.31788/RJC.2019.1245132
Purushotham E (2019b) Synthesis and effect of lattice strain on the Debye-Waller factors of zinc nanoparticles. Modern Chem 7(1):5–9. https://doi.org/10.11648/j.mc.2019.0701.12
Purushotham E (2019c) Preparation and characterization of cuinse2 Nano-particles. Int J Chem Stud 3(2):28–31.
Purushotham E (2019d) Effect of lattice strain on measured thermal properties of Fe nanoparticles—an X-ray diffraction study. Int J Fluid Mech Thermal Sci 5(3):63–66. https://doi.org/10.11648/j.ijfmts.20190503.11
Purushotham E (2019e) Preparation and characterization of cadmium metal nanoparticle-by X-ray study. Am J Quant Chem Molecular Spectrosc 3(2):37–40. https://doi.org/10.11648/j.ajqcms.20190302.12
Purushotham E (2019f) Synthesis, characterization, effect of Lattice strain on the Debye-Waller factor and Debye temperature of aluminium. Am J Nanosci 5(3):23–26. https://doi.org/10.11648/j.ajn.20190503.11
Purushotham E (2020a) Mechanical and thermal properties of strained metal nanopparticles prepared by ball milling method. Rasayan J Chem 13(4):1676–1680. https://doi.org/10.31788/RJC.2020.1345788
Purushotham EF (2020b) X-Ray Debye temperature study of Gruneisen constant of Hexagonal Phase Cu1-x-Znx alloys. In: IOP Conference Series: Materials Science and Engineering, (SCOPUS JOURNAL), 981 022085. No 1–9. https://doi.org/10.1088/1757-899X/981/2/022085
Purushotham E (2020c) Characterization of size dependent thermal properties in strained nanocrystalline powder using Williamson–Hall. IOP Conference Series: Materials Science and Engineering, (SCOPUS JOURNAL), 1757–8981, 981 022086, 1–6. https://doi.org/10.1088/1757-899X/981/2/022086
Purushotham E (2021a) Evaluation of Debye temperatures of α- phase copper–zinc alloys by using X-Ray diffraction method. Mater Today Proc 46 (part 12):5922–5926. https://doi.org/10.1016/j.matpr.2021.03.556
Purushotham E (2021b) Evaluation of particle size, lattice strain and estimation of Mean square amplitudes of vibration, Debye Waller factor and Debye temperature for metal oxide nanoparticles. Lecture Notes on Data Engineering and Communications Technologies 63 (ISBN 978-981-16-0080-7, 63, pp. No.297–304. https://doi.org/10.1016/j.matpr.2021.03.556
Purushotham E (2021c) X-Ray Determination of Debye temperature and microhardness of Some Hexagonal Close Packed Elements Re, Os and Tl. IOP Conference Series: Materials Science and Engineering, 1757–8981, 1119, 012001, pp 1–5, https://doi.org/10.1088/1757-899X/1119/1/012001
Purushotham E (2021d) Preparation and characterization of zinc nanoparticles: Ball milling and Hall-Williamson method. Mater Today Proc 47 (part 15):5391–5394. https://doi.org/10.1016/j.matpr.2021.06.142
Purushotham E (2021e) Root mean square amplitudes of vibration and associated Debye temperatures of beryllium, scandium and ruthenium. Materials Today Proc 47 (part 15):5034–5037. https://doi.org/10.1016/j.matpr.2021.04.616
Purushotham E, Gopi Krishna N (2010a) Mean square amplitudes of vibration and associated Debye temperatures of rhenium, osmium and thallium. Phys B: Condensed Matter 405(16):3308–3311.
Purushotham E, Gopi Krishna N (2010b) Effect of lattice strain on the Debye-waller factors of Mg, Zn and Cd. Ind J Phys 84(7):887–893.
Purushotham E, Gopi Krishna N (2014) Synthesis and characterization of Tungsten (W) nano particles. J Eng Sci Technol Rev (SCOPUS Journal) 7(1):41–44.
Purushotham E, Gopi Krishna N (2015) X-Ray determinationof crystallite size and effect. Chem Mater Res 2224–3224, 7(2):1–6
Rao CNR, Muller A, Cheetam AK (2004) The chemistry of nanomaterials : synthesis, properties and applications. Wiley, Weinheim
Sirdeshmukh DB, Sirdeshmukh L, Subhadra KG (2006) Micro-and macro-properties of solids. Springers Series in Materials Science, New York
Sirdeshmukh DB, Subhadra KG, Hussain KA, Gopi Krishna N, Raghavendra Rao B (1993) X-Ray study of strained CdTe powders. Cryst Res Technol 28:15
Yang Geng, Zhang-Yi Xie, Sai-Sheng Xu, Qing-Qing Sun, Shi-Jin Ding, Hong-Liang Luz, David Wei Zhang (2012) Effects of rapid thermal annealing on structural, luminescent, and electrical properties of Al-Doped ZnO films grown by atomic layer deposition. ECS J Solid State Sci Technol 1(3):N45–N48. https://doi.org/10.1149/2.015203jss
Vetelino JF, Gaur SP, Mitra SS (1972) Lattice dynamics of cesium chloride. Phys Rev B 5:2360
Wilson A.JC (1949) X-ray Optics. Methuen, London.
Xiaofei Zhao, Lei Wang, Xin Xu, Xiaodong Lei, Sailong Xu and Fazhi Zhang (2012), Interfaces, and Electrochemical Phenomena, AIChE Journal of Materials, Issue 2Volume 58, 573–582, https://doi.org/10.1002/aic.12597
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Endla, P. Synthesis and evaluation of ZnAl2O4 and Mn, Dy doped ZnAl2O4 powders by sol–gel and ball milling method. Chem. Pap. 76, 7327–7331 (2022). https://doi.org/10.1007/s11696-022-02409-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02409-4