Abstract
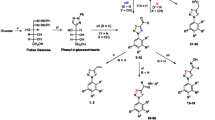
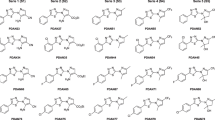
Chagas disease is caused by the etiological agent Trypanosoma cruzi that impacts negatively on society and mainly affects the poorest populations of the community. The treatment is restricted to two drugs that have been on the market since the 1960s: nifurtimox and benznidazole. However, both have a high incidence of unwanted side effects and low efficiency in the chronic phase of the disease. Therefore, in this context, the objective of this work was to synthesize chromenopyrazole derivatives and to evaluate their antiparasitic activity in vitro against the intracellular forms of T. Cruzi. Chromenopyrazoles are heterocyclic compounds having as a basic core a benzene ring fused to a pyran ring and a pyrazole ring, thus forming a tricyclic compound with a 6, 6, 5 arrangement. Reaction of 3-benzoyl-flavanone with hydrazine was expected to afford the target compounds, but for a similar synthetic route described in the literature, the proposed product was a pyrazole derivative in an open form that had not undergone the final conjugate addition step. Based on NMR and X-ray crystallography analysis, it has been demonstrated that a tricyclic chromenopyrazole is the correct structural representation for the product of this reaction. This study resulted in the synthesis of 15 novel chromenopyrazoles compounds displaying significant trypanocidal activity. The majority of the chromenepyrazoles satisfied Lipinski rules, without any violations, whilst only two compounds showed at least one violation of the rule, due to the log P being greater than 5.6. All chromenepyrazoles exhibited anti-T. cruzi activity, and improved potency was observed when comparing them to the precursor 3-benzoyl-flavanone. The introduction of an anisole moiety at the pyrazole ring and the inclusion of 3,4,5-trimethoxybenzene at the pyranone resulted in a doubling of potency and improvement in selectivity. The lead compound bearing methoxyl groups was the most active and displayed comparable anti-T. Cruzi activity to the control drug benznidazole. This result, once again, reinforces the same observations reported in the literature in which the introduction of the methoxy groups favoured either more active or more selective trypanocidal compounds.





Similar content being viewed by others
References
Andrade JS, Abreu LG, Junior PAS, Murta SMF, Taylor JG (2021) In vitro evaluation of synthetic flavones against trypanosoma cruzi. Rev Virtual Quim 13:146–155. https://doi.org/10.21577/1984-6835.20200136
Argolo AM, Felix M, Pacheco R, Costa J (2008) Doença de chagas e seus principais vetores no Brasil/ chagas disease and its main vectors in Brazil.Rio de Janeiro: Imperial Novo Milênio. https://doi.org/10.13140/2.1.1578.9449
Bezerra WS, Meneguetti DUO, Camargo LMA (2012) The pursuit of drugs for chagas disease treatment (american trypanosomiasis): 103 years of neglect. Saúde (Santa Maria) 38:9–20. https://doi.org/10.5902/223658344813
Brito ACF, Correa RS, Pinto AA, Matos MJS, Tenorio JC, Taylor JG, Cazati T (2018) Synthesis, crystal structure, photophysical properties and theoretical studies of a novel bis(phenylisoxazolyl) benzene derivative. J Mol Struct 1163:197–204. https://doi.org/10.1016/j.molstruc.2018.03.009
Capela R, Moreira R, Lopes F (2019) An overview of drug resistance im protozoal diseases. Mol Sci 20:17–23. https://doi.org/10.3390/ijms20225748
Chen Z, Wang Z, Su W (2012) Synthesis and antitumour activity of a novel class of flavanones: 1,4-diaryl-1,4-dihydrochromeno[4,3-c]pyrazoles. J Chem Res 1:45–48. https://doi.org/10.3184/174751912X13263828746643
Coelho GS, Andrade JS, Xavier VF, Junior PAS, Araujo BCR, Fonseca KS, Caetano MS, Murta SM, Vieira PM, Carneiro CM, Taylor JG (2019) Issue information. Chem Biol Drug Des 93:1–14. https://doi.org/10.1111/cbdd.13420
Diogo GM, Andrade JS, Junior PAS, Murta MF, Santos VMR, Taylor JG (2020) Trypanocidal activity of flavanone derivatives. Molecules 25:397. https://doi.org/10.3390/molecules25020397
Elias PR, Coelho GS, Xavier VF, Junior PAS, Romanha AJ, Murta SMF, Carneiro CM, Camilo NS, Hilario FF, Taylor JG (2016) Synthesis of xylitan derivatives and preliminary evaluation of in vitro trypanocidal activity. Molecules 21:1342. https://doi.org/10.3390/molecules21101342
Farrugia LJ (2012) WinGX and ORTEP for windows: an update. J Appl Crystallogr 45:849–854. https://doi.org/10.1107/S0021889812029111
Freymann DM, Wenck MA, Engel JC, Feng J, Focia PJ, Eakin AE, Craig SP (2000) Efficient identification of inhibitors targeting the closed active site conformation of the HPRT from Trypanosoma cruzi. Chem Biol 7:957–968. https://doi.org/10.1016/S1074-5521(00)00045-4
Kumbhare RM, Chinchkhede PW, Ingle VN (2000) Synthesis of Benzopyranopyrazoles. Asian J Chem 12:501–505
Lovering F (2013) Escape from flatland 2: complexity and promiscuity. Med Chem Comm 4(3):515–519. https://doi.org/10.1039/C2MD20347B
Lucio JG, Valencia MT, Domínguez AR, Peña HN, López BA, Pacheco JS, Domínguez CA, Valencia AT (2009) Selective inactivation of triosephosphate isomerase from trypanosoma cruzi by brevifolin carboxylate derivatives isolated from geranium bellum rose. Bioorg Med Chem Lett 19:5936–5939. https://doi.org/10.1016/j.bmcl.2009.08.055
Macrae CF, Sovago I, Cottrell SJ et al (2020) Mercury 4.0: from visualization to analysis, design and prediction. J Appl Crystallogr 53:226–235. https://doi.org/10.1107/S1600576719014092
Madhu G, Sudhakar M, Kumar K, Reddly GR, Sravani A, Ramakrish K, Prasadrao C (2017) Synthesis of pyrazole-substituted chromene analogues with selective ant-leukemic activity. Russ J Gen Chem 87:2421–2428. https://doi.org/10.1134/S1070363217100243
Marin-neto JA, Junior AR, Souza AS, Rassi A, Dias JCP (2014) Doença de chagas: moléstia negligenciada. São Paulo Atheneu Chapter 18:2–10
Menezes JCL, Vaz LBA, Abreu VPM, Silva FK, Fonseca KS, Carneiro CM, Taylor JG (2015) Synthesis and anti-trypanosoma cruzi activity of diaryldiazepines. Molecules 20:43–51. https://doi.org/10.3390/molecules20010043
Ministério da saúde (2020) Doença de Chagas. Ministério da saúde. To be found under http://www.saude.gov.br/saude-de-a-z/doenca-de-chagas. Published on 16/11/2020 and updated on 14/04/2021
Niquini FM, Machado PH, Rodrigues JHV, Pontes-Silva AV, Figueiredo RC, Silveira RG, Corrêa RS (2022) On the experimental and theoretical calculations of rotameric conformations of a new Schiff base derived from amantadine. J Mol Struct 1256:132489. https://doi.org/10.1016/j.molstruc.2022.132489
PAHO (2019) Chagas disease. To be found under http://www.paho.org/hq/index.php?option=com_content&view=article&id=13566:chagas-in-americas&Itemid=40721&lang=en. Updated in August 2019
Pietro OD, García EV, Taylor MC, Berenguer D, Viayna E, Lanzoni A, Sola I, Sayago H, Riera C, Fisa R, Close MV, Péreze B, Kelly JM, Lavilla R, Torrero DM (2015) Multicomponent reaction-based synthesis and biological evaluation of tricyclic heterofused quinolines with multi-trypanosomatid activity. Eur J Med Chem 105:120–137. https://doi.org/10.1016/j.ejmech.2015.10.007
Rodriguez SV, Guíñez RF, Matos MJ, Azar CO, Maya JD, Uriarte E, Santana L, Borges F (2015) Synthesis and trypanocidal properties of new coumarin-chalcone derivatives. Med Chem 5:173–177. https://doi.org/10.4172/2161-0444.1000260
Romanha AJ, Castro SL, Soeiro MC, Vieira JL, Ribeiro I, Talvani A, Bourdin B, Blum B, Olivieril B, ZaniI C, Spadafora C, Chiari E, Chatelain E, Chaves G, Calzada JE, Bustamante JM, Junior LHF, Romero LI, Bahia MT, Lotrowska M et al (2010) In vitro and in vivo experimental models for drug screening and development for chagas. Mem Inst Oswaldo Cruz 105:233–238
Rout SK, Guin S, Banerjee A, Khatun N, Gogoi A, Patel B (2013) Cu(II) catalyzed imine C-H functionalization leading to synthesis of 2,5-substituted 1,3,4-oxadiazoles. Org Lett 15:4106–4109. https://doi.org/10.1021/ol202409r
Sambaiah M, Raghavulu K, Shiva K, Yennam S, Behera MJ (2017) Synthesis of novel fused chromone-pyrimidine hybrids and 2,4,5-trisubstituted pyrimidine derivatives via ANRORC rearrangement. Chem 41:10020–10026. https://doi.org/10.1039/C7NJ01839H
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem 71:3–8. https://doi.org/10.1107/S2053229614024218
Silva ENS, Guimarães TT, Menna-Barreto RFS, Pinto MCFR, Simone CA, Pessoa C, Cavalcanti BC, Sabino JR, Andrade CKZ, Goulart MOF, Castro SL, Pinto AV (2010) The evaluation of quinonoid compounds against Trypanosoma cruzi: synthesis of imidazolic anthraquinones, nor-β-lapachone derivatives and β-lapachone-based 1,2,3-triazoles. Bioorg Med Chem 18:3224–3230. https://doi.org/10.1016/j.bmc.2010.03.029
Sinan (2019) Doença de Chagas aguda. To be found under http://www.portalsinan.saude.gov.br/doenca-de-chagas-aguda. Published on 08/03/2016 and updated on 24/01/2019
Souza AA, Xavier VF, Coelho GS, Junior PAS, Romanha AJ, Murta SMF, Carneiro CM, Taylor JG (2017) Synthesis of 3,5-diarylisoxazole derivatives and evaluation of in vitro trypanocidal activity. J Braz Chem Soc 29:269–277. https://doi.org/10.21577/0103-5053.20170137
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. Authors gratefully acknowledge the generous financial support from the Universidade Federal de Ouro Preto (UFOP), FAPEMIG and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). The authors thank the Program for Technological Development of Tools for Health-PDTIS-FIOCRUZ for use of its facilities (Chagas Disease Platform PlaBio Tc). The authors thank the Laboratório Multiusuário de Caracterização de Moléculas (UFOP) for Nuclear Magnetic Resonance service. The authors thank the IFSC-USP (E.E. Castellano and J. Honorato) for X-ray crystallography measurements. The authors would also like to thank the Laboratório Multiusuário de Proteômica e Biomoléculas (LMU-ProtBio), from Núcleo de Pesquisas em Ciências Biológicas, Universidade Federal de Ouro Preto, MG, Brazil, for providing the required equipment and technical expertise for sample processing and mass spectrometry analyses. Policarpo Ademar Sales Junior is research fellow supported by CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Andrade, J.S., Junior, P.A.S., Pereira, F.J. et al. Trypanocidal activity of chromenepyrazole derivatives. Chem. Pap. 76, 5827–5837 (2022). https://doi.org/10.1007/s11696-022-02283-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02283-0