Abstract
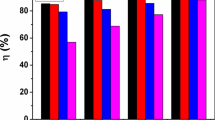
A Schiff base, 2-[(E)-(2,5-dimethoxybenzylidene) amino]-4-methylphenol (DMPC) was synthesized by condensation reaction of 2,5-dimethoxybenzaldehyde with 2-amino-4-methylphenol at 35 °C. The integrity of the synthesized compound was determined by spectroscopic techniques such as FT-IR, NMR, UV/visible and ESI–MS. Quality single crystals of the compound were also harvested and analyzed using X-ray diffraction (XRD) method. The XRD data reveal that the compound crystallized out in monoclinic crystal system with space group of C2/c and Z = 8-unit cell. The corrosion inhibition performance of the Schiff base on mild steel in 1.0 M HCl was evaluated using potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS), surface analysis and computational studies. The inhibition efficiency obtained from PDP and EIS at the optimum inhibitor concentration is 96.56% and 97.02%, respectively. Data from polarization measurement indicate that the compound acted as a mixed-type inhibitor in the tested media. The adsorption study carried out showed that Langmuir adsorption mechanism was followed since R2 value is very close to unity, and also, the slope of the graph approaches unity. The compound was found to adsorb on the mild steel surface by both physisorption and chemisorption adsorption mechanisms. The EDX spectra analysis shows that oxygen was eliminated from the mild steel surface while SEM result portrays significant reduction of mild steel corrosion in the presence of the studied inhibitor, compared with the blank. The results obtained from DFT calculations agree the experimental findings.











Similar content being viewed by others
References
Abdallah M, Zaafarany I, Fouda A, Abd El-Kader D (2012a) Inhibition of zinc corrosion by some benzaldehyde derivatives in HCl solution. J Mater Eng Perf 21:995–1002. https://doi.org/10.1007/s11665-011-9990-4
Abd-El-Nabey B, Abdel-Gaber A, Elewady G, Sadeek M, Abd-El-Rhman H (2012) Inhibitive action of benzaldehyde thiosemicarbazones on the corrosion of mild steel in 3 MH3po4. Int J Electrochem Sci 7:11718–11733. https://doi.org/10.1016/j.jtice.2014.10.024
Abd-El-Nabey B, Abdel-Gaber A, Elewady G, Sadeek M, Abd-El-Rhman H (2012) Inhibitive action of benzaldehyde thiosemicarbazones on the corrosion of mild steel in 3 MH3po4. Int J Electrochem Sci 7:11718–11733
Adejo S, Ekwenchib M, Gbertyoa J, Menengea T, Ogbodoc J (2014) Determination of adsorption Isotherm model best fit for methanol leaf extract of Securinega virosa as corrosion inhibitor for corrosion of mild steel in HCl. J Adv Chem 10:2737
Adejoro IA, Akintayo DC, Ibeji CU (2016) The efficiency of chloroquine as corrosion inhibitor for aluminium in 1M HCl solution: experimental and DFT study. Jordan J Chem 146:1–12
Afak S (2012) B, Duran, A. Yurt, G. Turkoglu. Corros Sci 54:251–259
Al-Amiery AA, Kadhum AAH, Mohamad AB, Junaedi S (2013) A novel hydrazinecarbothioamide as a potential corrosion inhibitor for mild steel in HCl. Materials 6:1420–1431
Alaneme KK, Olusegun SJ, Adelowo OT (2016) Corrosion inhibition and adsorption mechanism studies of Hunteria umbellata seed husk extracts on mild steel immersed in acidic solutions. Alex Eng J 55:673–681
Ansari F, Verma C, Siddiqui Y, Ebenso E, Quraishi M (2018) Volatile corrosion inhibitors for ferrous and non-ferrous metals and alloys: a review. Int J Corros Scale Inhib 7:126–150
Ashassi-Sorkhabi H, Shaabani B, Seifzadeh D (2005) Corrosion inhibition of mild steel by some Schiff base compounds in hydrochloric acid. Appl Surf Sci 239:154–164
Becke AD (1993) Becke’s three parameter hybrid method using the LYP correlation functional. J Chem Phys 98:5648–5652
Belakhdar A et al (2020) Computational and experimental studies on the efficiency of Rosmarinus officinalis polyphenols as green corrosion inhibitors for XC48 steel in acidic medium. Coll Surf A Physicochem Eng Aspects 606:125458
Benabid S, Douadi T, Issaadi S, Penverne C, Chafaa S (2017) Electrochemical and DFT studies of a new synthesized Schiff base as corrosion inhibitor in 1 M HCl. Measurement 99:53–63
Bingöl D, Zor S (2013) Optimization of the experimental variables influencing the corrosion rate of aluminum using response surface methodology. Corrosion 69(5):462–467. https://doi.org/10.5006/0382
Chaitra TK, Mohana KNS, Tandon HC (2015) Thermodynamic, electrochemical and quantum chemical evaluation of some triazole Schiff bases as mild steel corrosion inhibitors in acid media. J Mol Liq 211:1026–1038
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JA, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341
Dominic OO, Monday O (2016) Optimization of the inhibition efficiency of mango extract as corrosion inhibitor of mild steel in 1.0 M H2SO4using response surface methodology. J Chem Technol Metall. 51:302–314
Ezeorah JC et al (2018) Synthesis, characterization and computational studies of 3-{(E)-[(2-hydroxyphenyl) imino] methyl} benzene-1, 2-diol and molecular structure of its zwitterionic. J Mol Struct 1152:21–28
Ferkous H, Djellali S, Sahraoui R, Benguerba Y, Behloul H, Çukurovali A (2020) Corrosion inhibition of mild steel by 2-(2-methoxybenzylidene) hydrazine-1-carbothioamide in hydrochloric acid solution: Experimental measurements and quantum chemical calculations. J Mol Liq 307:112957
Finšgar M, Jackson J (2014) Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review. Corros Sci 86:17–41
Frisch M et al (2009) Gaussian 09, revision D 0.1. Gaussian Inc., Wallingford
Ghames A, Douadi T, Issaadi S, Sibous L, Alaoui KI, Taleb M, Chafaa S (2017) Theoretical and experimental studies of adsorption characteristics of newly synthesized schiff bases and their evaluation as corrosion inhibitors for mild steel in 1 M HCl. Int J Electrochem Sci 12:4867–4897
Ghasemi O, Ghadimi M, Ghasemi V (2014) Adsorption and inhibition effect of benzaldehyde Schiff bases on mild steel corrosion in 1 M HCl medium. J Dispers Sci Technol 35:1143–1154
Gürten AA, Keleş H, Bayol E, Kandemirli F (2015) The effect of temperature and concentration on the inhibition of acid corrosion of carbon steel by newly synthesized Schiff base. J Ind Eng Chem 27:68–78
Hamadi L, Mansouri S, Oulmi K, Kareche A (2018) The use of amino acids as corrosion inhibitors for metals: a review. Egypt J Pet 27:1157–1165
Ibeji CU, Adejoro IA, Adeleke BB (2015) A benchmark study on the properties of unsubstituted and some substituted polypyrrole. J Phys Chem Biophys 5(6):1–11
Ibeji CU et al (2021) Dehydroacetic acid-phenylhydrazone as a potential inhibitor for wild-type HIV-1 protease: structural, DFT, molecular dynamics, 3D QSAR and ADMET characteristics. Iran J Chem Chem Eng (IJCCE) 40:215–230. https://doi.org/10.30492/ijcce.2019.36702
Ichchou I, Larabi L, Rouabhi H, Harek Y, Fellah A (2019) Electrochemical evaluation and DFT calculations of aromatic sulfonohydrazides as corrosion inhibitors for XC38 carbon steel in acidic media. J Mol Struct 1198:126898
Jamil DM et al (2018) Experimental and theoretical studies of Schiff bases as corrosion inhibitors. Chem Cent J 12:1–9
Jensen F (2001) Polarization consistent basis sets: Principles. J Chem Phys 115:9113–9125
Madhifitri FG, Soenoko R, Suprapto A, Suprapto W (2018) Minimization of corrosion rate using surface response methodology. Eng. Rev 38(1):115–119
Masroor S (2017) Azomethine as potential corrosion inhibitor for different metals and alloys. J Bio-and Tribo-Corros 3:27
Njoku CN, Onyelucheya OE (2015) Response surface optimization of the inhibition efficiency of gongronema latifolium as an inhibitor for aluminium corrosion in HCl solutions. Int J Mater Chem 5:4–13
Nkuzinna OC, Menkiti MC, Onukwuli OD, Mbah GO, Okolo BI, Egbujor MC (2014) Application of factorial design of experiment for optimization of inhibition effect of acid extract of Gnetum africana on copper corrosion. Nat Res 5:299–307
Obasi L, Kaior G, Rhyman L, Alswaidan IA, Fun H-K, Ramasami P (2016) Synthesis, characterization, antimicrobial screening and computational studies of 4-[3-(4-methoxy-phenyl)-allylideneamino]-1, 5-dimethyl-2-phenyl-1, 2-dihydro-pyrazol-3-one. J Mol Struct 1120:180–186
Odewole OA et al (2021) Synthesis and anti-corrosive potential of Schiff bases derived 4-nitrocinnamaldehyde for mild steel in HCl medium: experimental and DFT studies. J Mol Struct 1223:129214
Odoemelam SA, Emeh UN, Eddy NO (2018) Experimental and computational chemistry studies on the removal of methylene blue and malachite green dyes from aqueous solution by neem (Azadirachta indica) leaves. J Taibah Univ Sci 12:255–265
Okey NC et al (2020) Evaluation of some amino benzoic acid and 4-aminoantipyrine derived Schiff bases as corrosion inhibitors for mild steel in acidic medium: Synthesis, experimental and computational studies. J Mol Liq 315:113773
Okpareke OC, Henderson W, Lane JR, Okafor SN (2020) Synthesis, structure, computational and molecular docking studies of asymmetrically di-substituted ureas containing carboxyl and phosphoryl hydrogen bond acceptor functional groups. J Mol Struct 1203:127360
Omoruwou F, Okewale A, Owabor C (2017) Statistical analysis of corrosion inhibition of water hyacinth on mild steel in an acidic medium. J Environ Anal Toxicol 7(2161–0525):1000481
Omotioma M, Onukwuli O (2017) Evaluation of Pawpaw leaves extract as anti-corrosion agent for Aluminium in Hydrochloric acid medium Nigerian. J Technol 36:496–504
Pandey A, Singh B, Verma C, Ebenso EE (2017) Synthesis, characterization and corrosion inhibition potential of two novel Schiff bases on mild steel in acidic medium. RSC Adv 7:47148–47163
Rigaku OD (2015) Crysalis Pro Rigaku Oxford diffraction, Yarnton, England
Saha SK, Ghosh P, Chowdhury AR, Samanta P, Murmu N, Lohar AK, Banerjee P (2014) Corrosion control of chrome steel ball in nitric acid medium using Schiff base ligand and corresponding metal complexes: a combined experimental and theoretical study. Can Chem Trans 2:381–402
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem 71:3–8
Sheldrick G (2015a) Acta Crystallogr Sect A Found Adv 3–8
Singh DK, Ebenso EE, Singh MK, Behera D, Udayabhanu G, John RP (2018) Non-toxic Schiff bases as efficient corrosion inhibitors for mild steel in 1 M HCl: electrochemical AFM, FE-SEM and theoretical studies. J Mol Liq 250:88–99
Thiruvengadam V, Kannan K, Zulfareen N (2015) Optimization of corrosion inhibition of mild steel by ethanol extract of Prosopis Juliflora in HCl medium using factorial analysis. Anal Bioanal Electrochem 7:701–718
Yadla SV, Sridevi V, Lakshmi M, Kumari SK (2012) A review on corrosion of metals and protection. Int J Eng Sci Adv Technol 2:637–644
Yusuf TL, Quadri TW, Tolufashe GF, Olasunkanmi LO, Ebenso EE, van Zyl WE (2020) Synthesis and structures of divalent Co, Ni, Zn and Cd complexes of mixed dichalcogen and dipnictogen ligands with corrosion inhibition properties: experimental and computational studies. RSC Adv 10:41967–41982
Zukal J et al (2016) White-nose syndrome without borders: Pseudogymnoascus destructans infection tolerated in Europe and Palearctic Asia but Not in North America. Sci Rep 6:1–17
Acknowledgements
EDA acknowledge the College of Science, Engineering and Technology, University of South Africa, Florida-South Africa, for providing financial assistance under the Postdoctoral Fellowship Scheme. CUI is thankful to CHPC (www.chpc.ac.za) and UKZN for operational and infrastructural support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Eze, S.I., Ibeji, C.U., Akpan, E.D. et al. Corrosion performance of Schiff base derived from 2, 5-dimethoxybenzyaldehyde: X-ray structure, experimental and DFT studies. Chem. Pap. 76, 5187–5200 (2022). https://doi.org/10.1007/s11696-022-02244-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02244-7