Abstract
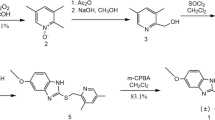
Bilastine is a new, well-tolerated, nonsedating H1 receptor antihistamine. Herein, we describe the synthesis of four new potential impurities of bilastine, namely 2-[4-(2-(4-(1-(2-methoxyethyl)benzimidazole-2-yl) piperidine-1-yl)ethyl) phenyl]-2-methylpropanoic acid (2) (methoxyethyl bilastine), 2-[4-(2-(4-(1-(2-(2-ethoxyethoxy)ethyl)benzimidazole-2-yl) piperidine-1-yl)ethyl)phenyl]-2-methyl propanoic acid (3) ((2-ethoxyethoxy)ethyl bilastine), 2-amino-2-methylpropyl 2-[4-(2-(4-(1-(2-ethoxyethyl)benzimidazole-2-yl) piperidine-1-yl)ethyl)phenyl]-2-methyl propanoate (4) (2-amino-2-methylpropyl ester of bilastine or bilastine open-ring ester) and 2-[4-(2-(4-(1-(2-ethoxyethyl)benzimidazole-2-yl] piperidine-1-yl) ethyl) phenyl]-N-(1-hydroxy-2-methylpropan-2-yl)-2-methylpropanamide (5) [N-(1-hydroxy-2-methyl-2-propanyl)amide of bilastine or bilastine open-ring amide]. Each (2, 3, 4 and 5) is an observed process-related impurity with a possible significant impact on the quality of the drug product. This work is useful for generic pharmaceutical industry for making impurity reference standards. Pharmacopeia is not available for bilastine; hence, the control of these impurities in the API below the threshold level is essential as per International Conference on Harmonization (ICH) recommendations.









Similar content being viewed by others
Abbreviations
- DCM:
-
Dichloromethane
- DIPEA:
-
N,N-diisopropylethylamine
- TBAB:
-
Tetrabutylammonium bromide
- NaCl:
-
Sodium chloride
- MTBE:
-
Methyl tertiary-butyl ether
- EDC·HCl:
-
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- Na2CO3 :
-
Sodium carbonate
- DMAP:
-
4-Dimethylaminopyridine
- NaOH:
-
Sodium hydroxide
- HCl:
-
Hydrochloric acid
- H2SO4 :
-
Sulfuric acid
- Boc2O:
-
Di-tert-butyl dicarbonate (Boc anhydride)
References
Bousquet J, Cauwenberge PV, Khaltaev N (2001) Allergic rhinitis and its impact on asthama. J Allergy Clin Immunol 108(5):S147-334. https://doi.org/10.1067/mai.2001.118891
Carter NJ (2012) Bilastine in allergic rhinitis and urticaria. Drugs 72(9):1257–1269. https://doi.org/10.2165/11209310-000000000-00000
Collier SJ, Wu X, Poh Z, Rajkumar GA, Yet L (2011) Alternative synthesis of bilastine. Synth Commun 41:1394–1402. https://doi.org/10.1080/00397911.2010.486506
Ding HX, Liu KK-C, Sakya SM, Flick AC, O’Donnell CJ (2013) Synthetic approaches to the 2011 new drugs. Bioorg Med Chem 21:2795–2825. https://doi.org/10.1016/j.bmc.2013.02.061
International Conference on Harmonization (ICH) Guidelines Q3A (R2): Impurities in New Drug Substances (2006).
Kaizhong M, Xin H (2017) Bilastine compound and preparation method. CN Patent: 104530002B
Lee C-H, Khoo J-H, Kwon K-C, Ju H (2013) Process for preparation of 2-methyl-2 phenylpropionic acid derivatives and novel intermediate compounds. US Patent: 8367704B2
Lei W, Ke L, Qian W, Weifeng L (2014) Preparation method of 2-(4-haloethyl) phenyl-2-methylpropionate and method for synthesizing bilastine. CN Patent: 102675101B
Lian L, Hua Y, Zhanxiao (2015) 2-phenylpropionate derivative and preparation method and use thereof. CN Patent: 105017211A
Liwei X, Limin D, Ning W, Dunchao T, Dechao Z, Dalong Z, Ke W (2016) Preparation method of bilastine. CN Patent: 104177331B
Orjales A, Rubio V, Bordell M (2004) Benzimidazole derivatives with antihistaminic activity. EP Patent: 0818454B
Pawankar R, Canonica GW, Holgate ST, Lockey RF (2011–2012) World Allergy Organization (WAO) white book on allergy: executive summary [online]. Available from https://www.worldallergy.org/publications/wao_white_book.pdf. Accessed 18 Aug 2021.
Ridolo E, Montagni M, Bonzano L, Incorvaia C, Canonica GW (2015) Bilastine: new insight into antihistamine treatment. Clin Mol Allergy 13:1. https://doi.org/10.1186/s12948-015-0008-x
Rongli Z, Xuan C, Chunguang S, Hongxin W (2014) Bilastine crystal formation and preparation method thereof. CN Patent: 103788062A
Srinivasan TR, Sajja E, Ghojala VR (2014) Novel process for the preparation of 2-[4-(2-{4-[l-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidinyl} ethyl) phenyl]-2-methylpropanoic acid. WO Patent: 2014188453A2
Tie L, Sufeng M, Qiqiang Y (2013) Preparation method of bilastine. CN Patent: 103214455A
Validation of Analytical Procedures, Text and Methodology Q2 (R1): (2006).
Wedi B (2008) Urticaria. J Dtsch Dermatol Ges 6(4):306–317. https://doi.org/10.1111/j.1610-0387.2008.06661.x
Wolthers OD (2013) Bilastine: a new nonsedating oral H1 antihistamine for treatment of allergic rhinoconjunctivitis and urticaria. Biomed Res Int. https://doi.org/10.1155/2013/626837
Yuenan Z, Qiqiang Y, Sufeng M, Jinmin W (2013) Method for preparing bilastine. CN Patent: 103351380A
Zezula J, Hajicek J (2014) A process for the preparation of a derivative of 2-methyl-2'-phenylpropionic acid using new intermediates. WO Patent: 2014026657A2
Zhen L, Chenggang Y, Yufa Y (2016) Preparation method of bilastine. CN Patent: 106146459A
Acknowledgements
The authors gratefully acknowledge Aurobindo Pharma Limited for supporting the work. The authors are also thankful to the Chemical Research Department and Analytical Research Department for the support and cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reddy, T.P., Dussa, N., Mamidi, S. et al. Identification and synthesis of potential impurities of bilastine drug substance. Chem. Pap. 76, 4137–4145 (2022). https://doi.org/10.1007/s11696-022-02157-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02157-5