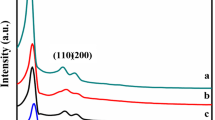
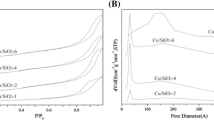
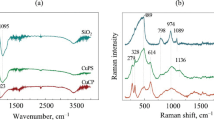
Abstract
Copper nanoparticles on silica support are cheap and effective catalysts for dehydrogenation of n-butanol, a biomass-derived building block which is increasingly used. Ultrasonically assisted Cu catalysts are prepared, characterized, and tested for the conversion of n-butanol to n-butanal. The best performance in terms of overall conversion of n-butanol and selectivity to butanal was offered by ultrasound-prepared process of the Cu nanoparticles. In conventional wet impregnation method, Cu nanoparticles on the surface of support tend to aggregate in some sort of cubic aggregates with the size of 4–5 µm and because of this, the percentage of the centers of silica (on which intermolecular dehydration reactions occur) is higher compared to Cu metallic centers (that promotes the dehydrogenation reaction). The ultrasound reduction stage used in the preparation of catalysts provides a better coverage of the catalyst surface by Cu nanoparticles that have a more uniform dimension, which offers a greater selectivity to butanal (more than 95%) and a negligible selectivity to butene.
Graphic Abstract











Similar content being viewed by others
Abbreviations
- (00):
-
Catalyst in simple form not exposed to pyridine
- B:
-
Brönsted
- B-Py:
-
Pyridine adsorbed on a Brönsted acid site
- CONV:
-
Conventional wet impregnated catalyst, 10%wt metal load
- Conv:
-
Conversion of n-butanol, %
- d BJH :
-
Average pore diameter computed by BJH from the desorption part of isotherm nm
- EDX:
-
Energy-dispersive X-ray
- FTIR:
-
Fourier transform infrared
- GHSV:
-
Gas hourly space velocity, s−1
- H-Py:
-
Pyridine adsorbed on a Brönsted-H type acid site
- L:
-
Lewis
- L-Py:
-
Pyridine adsorbed on a Lewis acid site
- m1 :
-
Mass of the unreacted n-butanol, mg
- m2 :
-
Mass of the n-butanol introduced in the system, mg
- m3 :
-
Mass of the n-butanal obtained, mg
- m4 :
-
Mass of the n-dibutyl ether obtained, mg
- mCAT :
-
Catalyst sample mass, g
- Mw1 :
-
Molecular weight of n-butanol, kg/kmol
- Mw2 :
-
Molecular weight of butanal, kg/kmol
- Mw3 :
-
Molecular weight of n-dibutyl ether, kg/kmol
- NA :
-
Avogadro’s number, sites/mol
- NPy :
-
Pyridine desorped from catalyst as a difference from the TGA curve for simple catalyst (00) and TGA curve for pyridine-exposed catalyst (Py), mol
- (Py):
-
Catalyst exposed to pyridine
- Py:
-
Pyridine
- S BET :
-
Specific surface area determined with BET method, m2/g
- Sel1 :
-
Selectivity to n-butanal (main product), %
- Sel2 :
-
Selectivity to n-dibutyl ether (secondary product), %
- Sel3 :
-
Selectivity to butene (secondary product), %
- SEM:
-
Scanning electron microscopy
- TGA:
-
Thermogravimetric analysis
- TPD:
-
Temperature programmed desorption
- V tp :
-
Total volume of pores, cm3/g
- UCu :
-
Uniformity with which the copper is distributed on the support surface
- US:
-
Ultrasound
- US10:
-
US impregnated catalyst, 10%wt metal load
- US20:
-
US impregnated catalyst, 20%wt metal load
- 2θ:
-
Diffraction angle
- ν:
-
FTIR wavenumber, cm−1
- Ψ:
-
Total surface acidity of the catalyst, sites/m2CAT
- BET:
-
Brunauer–Emmett–Teller
- BJH:
-
Barrett–Joyner–Halenda
- CAT:
-
Catalyst
References
Bang JH, Suslick KS (2010) Applications of ultrasound to the synthesis of nanostructured materials. Adv Mater 22(10):1039–1059. https://doi.org/10.1002/adma.200904093
Bankar SB, Survase SA et al (2013) Biobutanol: the outlook of an academic and industrialist. RSC Adv 3(47):24734. https://doi.org/10.1039/c3ra43011a
Calinescu I, Martin D et al (2014) Nanoparticles synthesis by electron beam radiolysis. Cent Eur J Chem 12(7):774–781. https://doi.org/10.2478/s11532-014-0502-x
Campanati M, Fornasari G et al (2003) Fundamentals in the preparation of heterogeneous catalysts. Catal Today 77(4):299–314. https://doi.org/10.1016/S0920-5861(02)00375-9
Chaturvedi S, Pragnesh ND et al (2012) Applications of nano-catalyst in new era. J Saudi Chem Soc 16(3):307–325. https://doi.org/10.1016/j.jscs.2011.01.015
Cherepanov PV, Andreeva DV (2017) Phase structuring in metal alloys: ULTRASOUND-assisted top-down approach to engineering of nanostructured catalytic materials. Ultrason Sonochem 35:556–562. https://doi.org/10.1016/j.ultsonch.2016.05.006
Cherepanov PV, Ashokkumar M et al (2014) Ultrasound assisted formation of Al-Ni electrocatalyst for hydrogen evolution. Ultrason Sonochem 23:142–147. https://doi.org/10.1016/j.ultsonch.2014.10.012
Cherepanov PV, Melnyk I et al (2015) Effect of high intensity ultrasound on Al3Ni2, Al3Ni crystallite size in binary AlNi (50 wt% of Ni) alloy. Ultrason Sonochem 23:26–30. https://doi.org/10.1016/j.ultsonch.2014.07.022
Chisega-Negrila C-G, Diacon A, et al. (2019) Conversion of n-butanol to n-butyraldehyde - screening of copper catalysts. U.P.B. Sci Bull Series B 81(2)
Dias HVR, Kharisov BI et al (2016) Study of high-power ultrasound-assisted processes using copper-containing precursors in aqueous media. Synth React Inorg Met-Org Nano-Met Chem 46(11):1605–1612. https://doi.org/10.1080/15533174.2015.1137033
Durre P (2011) Fermentative production of butanol-the academic perspective. Curr Opin Biotechnol 22(3):331–336. https://doi.org/10.1016/j.copbio.2011.04.010
Fitzgerald ME, Griffing V et al (1956) Chemical effects of ultrasonics—"Hot Spot" chemistry. J Chem Phys 25(5):926. https://doi.org/10.1063/1.1743145
Gabriëls D, Hernández WY et al (2015) Review of catalytic systems and thermodynamics for the Guerbet condensation reaction and challenges for biomass valorization. Catal Sci Technol 5:27. https://doi.org/10.1039/c5cy00359h
Gawande MB, Goswami A et al (2016) Cu and Cu-based nanoparticles: synthesis and applications in catalysis. Chem Rev 116(6):3722–3811. https://doi.org/10.1021/acs.chemrev.5b00482
Green EM (2011) Fermentative production of butanol–the industrial perspective. Curr Opin Biotechnol 22(3):337–343. https://doi.org/10.1016/j.copbio.2011.02.004
Halawy SA (2003) Unpromoted and K2O-promoted cobalt molybdate as catalysts for the decomposition of acetic acid. Monatsh Chem 134:371–380. https://doi.org/10.1007/s00706-002-0520-9
Herzberg G (1962) Molecular spectra and molecular structure II. Van Nostrand, New York
Keuler JN, Lorenzen L et al (2001) The dehydrogenation of 2-butanol over copper-based catalysts: optimising catalyst composition and determining kinetic parameters. Appl Catal A 218:171–180. https://doi.org/10.1016/S0926-860X(01)00639-1
Kuterasiński Ł, Podobiński J et al (2020) Reduction and oxidation of Cu species in Cu-Faujasites studied by IR spectroscopy. Molecules 25(20):4765. https://doi.org/10.3390/molecules25204765
Langford JI, Wilson A (1978) Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J Appl Crystallogr 11(2):102–113. https://doi.org/10.1107/s0021889878012844
Leonelli C, Mason TJ (2010) Microwave and ultrasonic processing: now a realistic option for industry. Br Chem Eng Process Technol 49(9):885–900. https://doi.org/10.1016/j.cep.2010.05.006
Levasseur A, Bahn O et al (2017) Assessing butanol from integrated forest biorefinery: a combined techno-economic and life cycle approach. Appl Energy 198:440–452. https://doi.org/10.1016/j.apenergy.2017.04.040
Liu K, Atiyeh HK et al (2014) Continuous syngas fermentation for the production of ethanol, n-propanol and n-butanol. Bioresour Technol 151:69–77. https://doi.org/10.1016/j.biortech.2013.10.059
Lomate S, Sultana A et al (2017) Effect of SiO2 support properties on the performance of Cu-SiO2 catalysts for the hydrogenation of levulinic acid to gamma valerolactone using formic acid as hydrogen source. Catal Sci Technol 7(14):3073–3083. https://doi.org/10.1039/C7CY00902J
Mascal M (2012) Chemicals from biobutanol: technologies and markets. Biofuels, Bioprod Bioref 6(4):483–493. https://doi.org/10.1002/bbb.1328
Mason TJ (1992) Industrial sonochemistry: potential and practicality. Ultrasonics 30:192–196. https://doi.org/10.1016/0041-624X(92)90072-T
Mason TJ (1997) Ultrasound in synthetic organic chemistry. Chem Soc Rev 26:443–451. https://doi.org/10.1039/CS9972600443
Mason TJ (1999) Sonochemistry: current uses and future prospects in the chemical and processing industries. Philos Trans R Soc A 357(1751):355–369. https://doi.org/10.1098/rsta.1999.0331
Mason T (1993) Advances in Sonochemistry, 31: 55938–476-X.
Mason T J (1996) Advances in sonochemistry, Elsevier Science ISBN: 1–55938–793–9
McNaught A D, Wilkinson A, (2021). Compendium of chemical terminology IUPAC recommendations, Gold Book.
Mekhemer GA, Halawy SA et al (2004) Qualitative and quantitative assessments of acid and base sites exposed on polycrystalline MgO surfaces: thermogravimetric, calorimetric, and in-Situ FTIR spectroscopic study combination. J Phys Chem B 108(35):13379–13386. https://doi.org/10.1021/jp040164s
Mitchell TM, Hammitt FG (1973) Asymmetric cavitation bubble collapse. J Fluids Eng 95(1):29–37. https://doi.org/10.1115/1.3446954
Munnik P, de Jongh PE et al (2015) Recent developments in the synthesis of supported catalysts. Chem Rev 115(14):6687–6718. https://doi.org/10.1021/cr500486u
Nanda S, Golemi-Kotra D et al (2017) Fermentative production of butanol: perspectives on synthetic biology. N Biotechnol 37(Pt B):210–221. https://doi.org/10.1016/j.nbt.2017.02.006
Osman AI, Abu-Dahrieh JK et al (2012) Effect of precursor on the performance of alumina for the dehydration of methanol to dimethyl ether. Appl Catal B 127:307–315. https://doi.org/10.1016/j.apcatb.2012.08.033
Perego C, Villa P (1997) Catalyst preparation methods. Catal Today 34:281–305. https://doi.org/10.1016/S0920-5861(96)00055-7
Saez V, Mason TJ (2009) Sonoelectrochemical synthesis of nanoparticles. Molecules 14(10):4284–4299. https://doi.org/10.3390/molecules14104284
Saleh IA, Vinatoru M et al (2016) A possible general mechanism for ultrasound-assisted extraction (UAE) suggested from the results of UAE of chlorogenic acid from Cynara scolymus L. (artichoke) leaves. Ultrason Sonochem 31:330–336. https://doi.org/10.1016/j.ultsonch.2016.01.002
Scherrer P, (1918) Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen, Mathematisch-Physikalische Klasse 1918 98–100 DOI: <http://eudml.org/doc/59018>.
Stavarache C, Vinatoru M et al (2004) Short-time sonolysis of chlorobenzene in the presence of Pd(II) salts and Pd(0). Ultrason Sonochem 11(6):429–434. https://doi.org/10.1016/j.ultsonch.2003.09.001
Suslick KS, Hyeon T et al (1995) Sonochemical synthesis of nanostructured catalysts. Mater Sci Eng A 204:186–192. https://doi.org/10.1016/0921-5093(95)09958-1
Wong KN, Coloson SD (1984) The FT-IR spectra of pyridine and pyridine-d5. J Mol Spectrosc 104(1):129–151. https://doi.org/10.1016/0022-2852(84)90250-9
Wongpisutpaisan N, Charoonsuk P et al (2011) Sonochemical synthesis and characterization of copper oxide nanoparticles. Energy Procedia 9:404–409. https://doi.org/10.1016/j.egypro.2011.09.044
Youssef I, Sall S et al (2019) Forward looking analysis approach to assess copper acetate thermal decomposition reaction mechanism. Am J Anal Chem 10:153–170. https://doi.org/10.4236/ajac.2019.105014
Zaki M, Hassan AM et al (2001) In situ FTIR spectra of pyridine adsorbed on SiO2–Al2O3, TiO2, ZrO2 and CeO2: general considerations for the identification of acid sites on surfaces of finely divided metal oxides. Colloids Surf, A 190:261–274
Acknowledgements
The authors acknowledge the financial support received from the mir, Action 1.1.4: Attracting high-level personnel from abroad in order to enhance the RD capacity, project: P_37_471, “Ultrasonic/Microwave Nonconventional Techniques as new tools for nonchemical and chemical processes,” financed by contract: 47/05.09.2016. The authors have no conflict of interest to declare that are relevant to the content of this article.
Funding
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chisega-Negrilă, CG., Diacon, A., Călinescu, I. et al. On the ultrasound-assisted preparation of Cu/SiO2 system as a selective catalyst for the conversion of biobutanol to butanal. Chem. Pap. 76, 1443–1455 (2022). https://doi.org/10.1007/s11696-021-01945-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01945-9