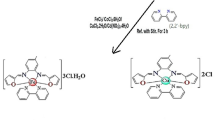
Abstract
The ligation behavior of the chalcone ligand namely (E)-3-(4-hydroxy-3-methoxyphenyl) acrylic acid (ferulic acid) (FA) toward the Cu(II) and Zn(II) ions was determined. The structure of the isolated solid complexes was elucidated by elemental analyses, spectral techniques (IR, UV–Vis, 13C– and 1H-NMR spectra) as well as the conductance measurements and thermal analyses. UV–Vis spectra and magnetic moments had suggested square planar and tetrahedral stereochemistry for Cu(II) and Zn(II) complexes, respectively. The kinetic and thermodynamic parameters for some selected decomposition steps have been calculated. Two precise and sensitive spectrophotometric methods were utilized to determine Zn(II) and Cu(II) complexes with ferulic acid using a micellar media of cetylpyridinium bromide (CPB) and sodium lauryl sulfate (SLS) with an absorption maxima of 430 and 465 nm for Zn(II) and Cu(II), respectively. Various analytical conditions, for example, the concentration of the reagent, temperature, the sequence and timing of addition were also looked into. Under optimum conditions, the complexes exhibited good linearity in concentration range of 2.0–70.0 and 4.0–140.0 µg mL−1; molar absorptivities 1.3161 × 104 and 8.826 × 103 L mol−1 cm−1; and Sandell’s sensitivity 0.00496 and 0.00719 µg cm−2 for the proposed methods of Zn(II) and Cu(II), respectively. The complexes ratio was found to be 1:2 [Zn(II):FA or Cu(II):FA] and the stability constants were 2.771 × 105 and 2.826 × 105, respectively. Finally, the newly synthesized complexes were shown potent antimicrobial activity.













Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Abd El-wahaab B, Elgendy K, El-didamony A (2020) Synthesis and characterization of new azo-dye reagent and using to spectrophotometric determination of samarium(III) in some industrial and blood samples. Chem Pap 74:1439–1448. https://doi.org/10.1007/s11696-019-01000-8
Admasu D, Reddy DN, Mekonnen KN (2016) Trace determination of zinc in soil and vegetable samples by spectrophotometry using pyridoxal thiosemicarbazone and 2-acetyl pyridine thiosemicarbazone. Cogent Chem 2:1249602–1249615. https://doi.org/10.1080/23312009.2016.1249602
Ahmed MJ, Zannat T (2012) A Simple spectrophotometric method for the determination of copper in some real, environmental, biological, food and soil samples using salicylaldehyde benzoyl hydrazone. Pak J Anal Environ Chem 13:22–35
Alabidi HM, Farhan AM, Al-Rufaie MM (2021) Spectrophotometric determination of Zn(II) in pharmaceutical formulation using a new azo reagent as derivative of 2-napthol. Curr Appl Sci Technol 21:176–187
Alharthi SS, Al-Saidi HM (2020) Spectrophotometric determination of trace concentrations of copper in waters using the chromogenic reagent 4-amino-3-mercapto-6-[2-(2-Thienyl)Vinyl]-1,2,4-Triazin-5(4H)-one: synthesis, characterization, and analytical applications. Appl Sci 10:3895–3911. https://doi.org/10.3390/app10113895
Babayeva K, Demir S, Andac M (2017) A novel spectrophotometric method for the determination of copper ion by using a salophen ligand, N, N′ -disalicylidene-2,3-diaminopyridine. J Taibah Univ Sci 11:808–814. https://doi.org/10.1016/j.jtusci.2017.02.001
Beecher DJ, Wong AC (1994) Identification of hemolysin BL-producing Bacillus cereus isolates by a discontinuous hemolytic pattern in blood agar. Appl Environ Microbial 60:1646–1651. https://doi.org/10.1128/aem.60.5.1646-1651.1994
Bower VE, Bates RG (1955) pH values of the Clark and Lubs buffer solutions at 25 °C. J Res Natl Bur Stand 55:197–200
Britton HTS (1952) Hydrogen ions, 4th edn, vol 28. London, Chapman and Hall, pp 359–364
Elgendy K, El-didamony A, Abd El-wahaab B (2020a) Analytical applications using spectrophotometric technique for the determination of uranium(VI), samarium(III) and cerium(III) by new organic reagent. J Iran Chem Soc 17:1317–1327. https://doi.org/10.1007/s13738-020-01856-8
Elgendy K, El-didamony A, Zaky M, Abd El-Wahaab B (2020) Spectrophotometric determination of cerium(III) in some industrial and plant samples using new synthesized azo-dye reagent: synthesis and characterization. Egypt. J. Chem. 63:2405–2417. https://doi.org/10.21608/ejchem.2019.16914.2029
El-Shwiniy WH, Shehab WS, Zordok WA (2020) Spectral, thermal, DFT calculations, anticancer and antimicrobial studies for bivalent manganese complexes of pyrano [2,3-d]pyrimidine derivatives. J Mol Struct 1199:126993–127042. https://doi.org/10.1016/j.molstruc.2019.126993
Fabre PL, Reynes O (2010) Determination of copper and zinc in brass: two basic methods. J Chem Educ 87:836–837. https://doi.org/10.1021/ed100259b
Frederickson CF, Koh JY, Bush AI (2005) The Neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–453. https://doi.org/10.1038/nrn1671
Geary WJ (1971) The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord Chem Rev 7:81–122. https://doi.org/10.1016/S0010-8545(00)80009-0
Genova G, Iacopini P, Baldi M, Ranieri A, Storchi P, Sebastiani L (2012) Temperature and storage effects on antioxidant activity of juice from red and white grapes. Int J Food Sci Technol 47:13–23. https://doi.org/10.1111/j.1365-2621.2011.02801.x
Horstkotte B, Alexovic M, Maya F, Duarte CM, Andruch V, Cerda V (2012) Automatic determination of copper by in-syringe dispersive liquid–liquid microextraction of its bathocuproine-complex using long path-length spectrophotometric detection. Talanta 99:349–356. https://doi.org/10.1016/j.talanta.2012.05.063
Jiaa H, Liu W, Li N, Wang J, Song Y (2020) Spectrophotometric determination of copper(II) in water based on fluorescein diacetate. J Anal Chem 75:330–342. https://doi.org/10.1134/s1061934820030089
Job P (1928) Formation and stability of inorganic complexes in Solution. Ann Chim 9:113–203
Kalinowska M, Piekut J, Bruss A, Follet C, Sienkiewicz-Gromiuk J, Swisłocka R, Rzaczynska Z, Lewandowski W (2014) Spectroscopic (FT-IR, FT-Raman, 1H, 13C NMR, UV/VIS), thermogravimetric and antimicrobial studies of Ca(II), Mn(II), Cu(II), Zn(II) and Cd(II) complexes of ferulic acid. Spectrochim Acta Part A 122:631–638. https://doi.org/10.1016/j.saa.2013.11.089
Kannan D, Arumugham MN (2013) Synthesis, characterization, DNA-binding studies and antimicrobial activity of copper (II) complex with 1, 10-phenanthroline, L-tyrosine and urea as ligands. Int J Inorg Bioinorg Chem 3:8–15
Lurie JU (1978) Handbook of analytical chemistry, 2nd edn. Mir publishers, Moscow
Lutfullah SS, Rahman N, Azmi SNH, Iqbal B, Amburk MIBB, Al Barwani ZMH (2010) UV spectrophotometric determination of Cu(II) in synthetic mixture and water samples. J Chin Chem Soc 57:622–631
Mohamed GG, Sharaby CM (2007) Metal complexes of Schiff base derived from sulphametrole and o-vanilin: synthesis, spectral, thermal characterization and biological activity. Spectrochim Acta A 66:949–958. https://doi.org/10.1016/j.saa.2006.04.033
Najim SS, Hameed MA, Al-Shakban MA, Fandi TS (2020) Spectrophotometric determination of zinc in pharmaceutical medication samples using 8-Hydroxyquinoline reagent. Int J Chem 12:29–36. https://doi.org/10.5539/ijc.v12n1p29
Okulik N, Jubert AH (2005) Theoretical analysis of the reactive sites of non–steroidal anti–inflammatory drugs. Internet Electron J Mol Des 4:17–30
Paiva LB, Goldbeck R, Santos WD, Squina FM (2013) Ferulic acid and derivatives: molecules with potential application in the pharmaceutical field. Braz J Pharm Sci 49:395–411. https://doi.org/10.1590/S1984-82502013000300002
Park CI, Huang HZ, Cha KW (2001) Spectrophotometric determination of uranium(VI) with pyrocatechol violet in surfactant media. Bull Korean Chem Soc 22:84–86
Raafid E, Al-Da’amy MA, Kadhim SH (2020) Spectrophotometric determination of Cu(II) in analytical sample using a new chromogenic reagent (HPEDN). Indones. J Chem 20:1080–1091. https://doi.org/10.22146/ijc.47894.
Rahmani M, Habibi M, NiAZi A (2015) Simultaneous spectrophotometric determination of Zinc and Copper with 4-(2-thiazolylazo) resorcinol using parallel factor analysis (PARAFAC), partial least squares (PLS) and orthogonal signal correction- partial least squares (OSC-PLS). Sci J 36:1601–1608
Reddy PNK, Reddy GT, Kumar SD, Reddy AVR, Parveen SN, Reddy NCG (2016) Spectrophotometric determination of Zn(II) in food and water samples using 2-hydroxy-N’-(1-(pyridin-2-yl)ethylidene) benzohydrazide as a sensitive and selective analytical reagent. Der Pharm Lett 8:251–259
Shiyi Ou, Kwok K-C (2004) Review Ferulic acid: pharmaceutical functions, preparation and applications in foods. J Sci Food Agric 84:1261–1269. https://doi.org/10.1002/jsfa.1873
Souza JC, Toci AT, Beluomini MA, Eiras SP (2016) Spectrophotometric determination of copper(II) in sugarcane spirit using 1-(2-pyridylazo)-2-naphthol and a homogeneous ternary mixture of the solvents water, ethanol and methyl isobutyl ketone. Rev Virtual Quim 8:687–701. https://doi.org/10.5935/1984-6835.20160052
Tirmizi SA, Wattoo FH, Wattoo MHS, Sarwar S, Memon AN, Ghangro AB (2012) Spectrophotometric study of stability constants of cimetidine–Ni(II) complex at different temperatures. Arab J Chem 5:309–314. https://doi.org/10.1016/j.arabjc.2010.09.009
Veitía MSI, Dumas F, Morgant G, Sorenson JRJ, Frapart Y (2009) Synthesis, structural analysis and anticonvulsant activity of a ternary Cu (II) mononuclear complex containing 1,10-phenanthroline and the leading antiepileptic drug valproic acid. Biochimie 91:1286–1293. https://doi.org/10.1016/j.biochi.2009.06.015
Wang Y, Chen X, Huang Z, Chen D, Yu B, Yu J, Chen H, He J, Luo Y, Zheng P (2020) Dietary ferulic acid supplementation improves antioxidant capacity and lipid metabolism in weaned piglets. Nutrients 12:3811–3821. https://doi.org/10.3390/nu12123811
Zaky RR, Yousef TA (2011) Spectral, magnetic, thermal, molecular modelling, ESR studies and antimicrobial activity of (E)-3-(2-(2-hydroxybenzylidene) hydrazinyl)-3-oxo-n(thiazole-2-yl)propanamide complexes. J Mol Struct 1002:76–85. https://doi.org/10.1016/j.molstruc.2011.06.050
Zdunska K, Dana A, Kolodziejczak A, Rotsztejn H (2018) Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Physiol 31:332–336. https://doi.org/10.1159/000491755
Zordok WA, Sadeek SA, El-Shwiniy WH (2012) Spectroscopic, thermal analysis, and antimicrobial evaluation of new Y(III), Zr(IV), and U(VI) ibuprofen complexes. J Coord Chem 65:353–369. https://doi.org/10.1080/00958972.2011.654203
Acknowledgements
The authors extend their appreciation to Zagazig University and University of Bisha.
Author information
Authors and Affiliations
Contributions
BAE contributed to conceptualization and funding acquisition; BAE and WSS contributed to investigation; WSS contributed to methodology; BAE, WSS and WHE contributed to project administration, resources, software, and supervision; WHE contributed to validation and visualization; BAE, WHE and WSS contributed to writing—original draft and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Informed consent
All authors consent to publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abd El-wahaab, B., Shehab, W.S. & El-Shwiniy, W.H. Synthesis, spectrophotometric, spectroscopic, microbial studies and analytical applications of Cu(II) and Zn(II) complexes of chalcone ligand. Chem. Pap. 76, 931–944 (2022). https://doi.org/10.1007/s11696-021-01916-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01916-0