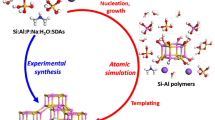
Abstract
2D minerals are among key elements of advanced systems, but the need for understanding their interactions/reactions with materials and systems in which they are involved necessitates tracking their molecular and atomic monitoring. Zeolitic structures are microporous materials formed in the nature through volcanic activities or synthesis. Because of their outstanding physicochemical properties like cation exchange capacity and excellent adsorption properties, zeolites have found application in diverse chemical processes, e.g., gas adsorption, water purification, and wastewater treatment. Prediction of zeolite performance for a targeted application saves time and expense as such projection could lead to the synthesis of optimum zeolite with adjusted properties. This review paper aims at encapsulating the latest findings on the use of 2D zeolite adsorbents studying three eminent molecular simulation techniques, namely molecular dynamics simulation, density functional theory, and Monte Carlo. Zeolites with precision structures and cost-efficiency for adsorption together with their adsorption capacity were correspondingly discussed in this review. Information gleaned from published reports on simulating zeolites’ adsorption properties could bridge with a brief comparison between the techniques mentioned to pave the way for scientists and industries to find the ideal method to predict zeolites performance and select the appropriate zeolite structure for the on-demand application.











Similar content being viewed by others
Availability of data and material
Available by the corresponding author per request through the email (amin.hamed.m@gmail.com)
References
Abdel Moamen OA, Ismail IM, Abdelmonem N, Abdel Rahman RO (2015) Factorial design analysis for optimizing the removal of cesium and strontium ions on synthetic nano-sized zeolite. J Taiwan Inst Chem Eng 55:133–144
Abdelrasoul A, Zhang H, Cheng C-H, Doan H (2017) Applications of molecular simulations for separation and adsorption in zeolites. Microporous Mesoporous Mater 242:294–348
Akten ED, Siriwardane R, Sholl DS (2003) Monte Carlo simulation of single-and binary-component adsorption of CO2, N2, and H2 in zeolite Na-4A. Energy Fuels 17:977–983
Alexopoulos K, Lee M-S, Liu Y, Zhi Y, Liu Y, Reyniers M-FO et al (2016) Anharmonicity and confinement in zeolites: structure, spectroscopy, and adsorption free energy of ethanol in. J Phys Chem C 120:7172–82
Aliano A, Cicero G (2012) Ab Initio DFT simulations of nanostructures. In: Bhushan B (ed) Encyclopedia of nanotechnology. Springer, Netherlands, Dordrecht, pp 11–17
Alver E, Metin AÜ (2012) Anionic dye removal from aqueous solutions using modified zeolite: adsorption kinetics and isotherm studies. Chem Eng J 200:59–67
Arya G, Maginn EJ, Chang H-C (2001) Effect of the surface energy barrier on sorbate diffusion in AlPO4-5. J Phys Chem B 105:2725–2735
Bacakova L, Vandrovcova M, Kopova I, Jirka I (2018) Applications of zeolites in biotechnology and medicine–a review. Biomaterials Science 6:974–989
Bai P, Haldoupis E, Dauenhauer PJ, Tsapatsis M, Siepmann JI (2016) Understanding diffusion in hierarchical zeolites with house-of-cards nanosheets. ACS Nano 10:7612–7618
Bandura L, Franus M, Józefaciuk G, Franus W (2015) Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 147:100–107
Bates SP, van Well WJ, van Santen RA, Smit B (1996) Location and conformation of n-alkanes in zeolites: an analysis of configurational-bias Monte Carlo calculations. J Phys Chem 100:17573–17581
Beerdsen E, Dubbeldam D, Smit B (2006) Loading dependence of the diffusion coefficient of methane in nanoporous materials. J Phys Chem B 110:22754–22772
Bensiradj NEH, Timón V, Boussessi R, Dalbouha S, Senent ML (2019) DFT studies of single and multiple molecular adsorption of CH4, SF6 and H2O in Zeolitic-Imidazolate Framework (ZIF-4 and ZIF-6). Inorg Chim Acta 490:272–281
Bhattacharya A, Naiya T, Mandal S, Das S (2008) Adsorption, kinetics and equilibrium studies on removal of Cr (VI) from aqueous solutions using different low-cost adsorbents. Chem Eng J 137:529–541
Biel O, Rożek P, Florek P, Mozgawa W, Król M (2020) Alkaline activation of kaolin group minerals. Crystals 10:268
Borah BJ, Jobic H, Yashonath S (2010) Levitation effect in zeolites: quasielastic neutron scattering and molecular dynamics study of pentane isomers in zeolite NaY. J Chem Phys 132:144507
Bu L, Nimlos MR, Robichaud DJ, Kim S (2017) Diffusion of biomass pyrolysis products in H-ZSM-5 by molecular dynamics simulations. J Phys Chem C 121:500–510
Burriesci N, Crisafulli ML, Saija LM, Polizzotti G (1983) Hydrothermal synthesis of zeolites from rhyolitic pumice of different geological origins. Mater Lett 2:74–78
Byrappa K, Yoshimura, M (2001) Hydrothermal synthesis and growth of zeolites. In: handbook of hydrothermal technology. University of Mysore Manasagangotri Mysore, India and Tokyo Institute of Technology, Elsevier, pp 315–414
Calleja G, Pau J, Calles J (1998) Pure and multicomponent adsorption equilibrium of carbon dioxide, ethylene, and propane on ZSM-5 zeolites with different Si/Al ratios. J Chem Eng Data 43:994–1003
Castillo J, Vlugt T, Dubbeldam D, Hamad S, Calero S (2010) Performance of chiral zeolites for enantiomeric separation revealed by molecular simulation. J Phys Chem C 114:22207–22213
Chanajaree R, Bopp PA, Fritzsche S, Kärger J (2011) Water dynamics in chabazite. Microporous Mesoporous Mater 146:106–118
Chandrakumar K, Pal S (2002) DFT and local reactivity descriptor studies on the nitrogen sorption selectivity from air by sodium and calcium exchanged zeolite-A. Colloids Surf A: Physicochem Eng Asp 205:127–38
Chatterjee A, Iwasaki T (1999) A novel approach using DFT to explain the selective permeation of small gaseous molecules through Y-type zeolite membrane. J Phys Chem A 103:9857–9863
Chebbi M, Chibani S, Paul J-F, Cantrel L, Badawi M (2017) Evaluation of volatile iodine trapping in presence of contaminants: a periodic DFT study on cation exchanged-faujasite. Microporous Mesoporous Mater 239:111–122
Chen X, Shen B, Sun H (2018) Ion-exchange modified zeolites X for selective adsorption desulfurization from Claus tail gas: experimental and computational investigations. Microporous Mesoporous Mater 261:227–236
Combariza AF, Sastre G (2011) Influence of zeolite surface in the sorption of methane from molecular dynamics. J Phys Chem C 115:13751–13758
Cosoli P, Ferrone M, Pricl S, Fermeglia M (2007) Grand canonical Monte-Carlo simulations for VOCs adsorption in non-polar zeolites. Int J Environ Technol Manage 7:228–243
Cosoli P, Ferrone M, Pricl S, Fermeglia M (2008) Hydrogen sulphide removal from biogas by zeolite adsorption: part I GCMC molecular simulations. Chem Eng J 145:86–92
Cundy CS, Cox PA (2005) The hydrothermal synthesis of zeolites: precursors, intermediates and reaction mechanism. Microporous Mesoporous Mater 82:1–78
Czuma N, Baran P, Franus W, Zabierowski P, Zas bska KJAS Technology (2019) Synthesis of zeolites from fly ash with the use of modified two-step hydrothermal method and preliminary SO2 sorption tests. Adsorp Sci Technol 37:61–76
De Wispelaere K, Ensing B, Ghysels A, Meijer EJ, Van Speybroeck V (2015) Complex reaction environments and competing reaction mechanisms in zeolite catalysis: insights from advanced molecular dynamics. Chem-A Eur J 21:9385–9396
Demontis P, Gulín-González J, Masia M, Suffritti GB (2010) The behaviour of water confined in zeolites: molecular dynamics simulations versus experiment. J Phys: Cond Matter 22:284106
Dempsey E, Kühl G, Olson DH (1969) Variation of the lattice parameter with aluminum content in synthetic sodium faujasites. Evidence for ordering of the framework ions. J Phys Chem 73:387–90
DING J, HU Y, YANG X, YANG J (2008) Monte Carlo simulation of water adsorption in ZSM-5 zeolite [J]. J Chem Indus Eng (China) 9
Drioli E, Giorno L (2016) Encyclopedia of Membranes [electronic resource]/edited by Enrico Drioli, Lidietta Giorno, Berlin, Heidelberg: Springer Berlin Heidelberg: Imprint: Springer.
Dyer A, Tangkawanit S, Rangsriwatananon K (2004) Exchange diffusion of Cu2+, Ni2+, Pb2+ and Zn2+ into analcime synthesized from perlite. Microporous Mesoporous Mater 75:273–279
Fellah MF (2017) A DFT study of hydrogen adsorption on Be, Mg and Ca frameworks in erionite zeolite. Appl Surf Sci 394:9–15
Ferreira ML, Al-Bogami SA, de Lasa HI (2016) Self diffusivity of n-dodecane and benzothiophene in ZSM-5 zeolites. its significance for a new catalytic light diesel desulfurization process. Int J Chem Reactor Eng 14:737–48
Fischer M, Bell RG (2014) Cation-exchanged SAPO-34 for adsorption-based hydrocarbon separations: predictions from dispersion-corrected DFT calculations. Phys Chem Chem Phys 16:21062–21072
Fu J, Feng X, Liu Y, Yang C (2017) Effect of pore confinement on the adsorption of mono-branched alkanes of naphtha in ZSM-5 and Y zeolites. Appl Surf Sci 423:131–138
Garcia G, Cardenas E, Cabrera S, Hedlund J, Mouzon J (2016) Synthesis of zeolite Y from diatomite as silica source. Microporous Mesoporous Mater 219:29–37
Gee JA, Chung J, Nair S, Sholl DS (2013) Adsorption and diffusion of small alcohols in zeolitic imidazolate frameworks ZIF-8 and ZIF-90. J Phys Chem C 117:3169–3176
Ghorbanzadeh Ahangari M, Hamed Mashhadzadeh A (2020) Density functional theory based molecular dynamics study on hydrogen storage capacity of C24, B12N12, Al12 N12, Be12O12, Mg12O12, and Zn12O12 nanocages. Int J Hydrogen Energy 45:6745–6756
Ghysels A, Moors SL, Hemelsoet K, De Wispelaere K, Waroquier M, Sastre G et al (2015) Shape-selective diffusion of olefins in 8-ring solid acid microporous zeolites. J Phys Chem C 119:23721–23734
Gomes EO, Fabris GSL, Ferrer MM, Motta FV, Bomio MRD, Andres J et al (2019) Computational procedure to an accurate DFT simulation to solid state systems. Comput Mater Sci 170:109176
Granato MA, Vlugt TJ, Rodrigues AE (2007) Molecular simulation of propane− propylene binary adsorption equilibrium in zeolite 4A. Ind Eng Chem Res 46:321–328
Gualtieri AF (2001) Synthesis of sodium zeolites from a natural halloysite. Phys Chem Miner 28:719–728
Gupta KM, Qiao Z, Zhang K, Jiang J (2016) Seawater pervaporation through zeolitic imidazolate framework membranes: atomistic simulation study. ACS Appl Mater Interfaces 8:13392–13399
Hamed Mashhadzadeh A, Fathalian M, Ghorbanzadeh Ahangari M, Shahavi MHDFT (2018a) Study of Ni, Cu, Cd and Ag heavy metal atom adsorption onto the surface of the zinc-oxide nanotube and zinc-oxide graphene-like structure. Mater Chem Phys 220:366–373
Hamed Mashhadzadeh A, Ghorbanzadeh Ahangari M, Salmankhani A, Fataliyan M (2018b) Density functional theory study of adsorption properties of non-carbon, carbon and functionalized graphene surfaces towards the zinc and lead atoms. Physica E 104:275–285
Hoffman A, DeLuca M, Hibbitts D (2019) Restructuring of MFI framework zeolite models and their associated artifacts in density functional theory calculations. J Phys Chem C 123:6572–6585
Hu Z, Chen Y, Jiang J (2011) Zeolitic imidazolate framework-8 as a reverse osmosis membrane for water desalination: insight from molecular simulation. J Chem Phys 134:134705
Hussain I, Titiloye J (2005) Molecular dynamics simulations of the adsorption and diffusion behavior of pure and mixed alkanes in silicalite. Microporous Mesoporous Mater 85:143–156
Jabraoui H, Hessou E, Chibani S, Cantrel L, Lebègue S, Badawi M (2019) Adsorption of volatile organic and iodine compounds over silver-exchanged mordenites: a comparative periodic DFT study for several silver loadings. Appl Surf Sci 485:56–63
Jafari L, Moradi H, Tavan Y (2020) A theoretical and industrial study of component co-adsorption on 3A zeolite: an industrial case. Chem Pap 74:651–661
Jamali SH, Vlugt TJ, Lin L-C (2017) Atomistic understanding of zeolite nanosheets for water desalination. J Phys Chem C 121:11273–11280
Karami Z, Mashhadzadeh AH, Habibzadeh S, Ganjali MR, Ghardi EM, Hasnaoui A et al (2021b) Atomic simulation of adsorption of SO 2 pollutant by metal (Zn, Be)-oxide and Ni-decorated graphene: a first-principles study. J Mol Model 27:1–10
Karami Z, Hamed Mashhadzadeh A, Shahmoradi A, Ganjali MR, Vatanpour V, Esmaeili A et al (2021) Lead adsorption onto Ni- and Pt-decorated nano γ-alumina: a first-principles study. J Molecul Liquids 337:116349
Khadem SSM, Mashhadzadeh AH, Habibzadeh S, Munir MT, Lima EC, Saeb MR (2021) A theoretical probe into the effects of material and operational variables on water purification with zeolite membranes. Microporous Mesoporous Mater 320:111070
Khalili R, Zarrintaj P, Jafari SH, Vahabi H, Saeb MR (2020) Electroactive poly (p-phenylene sulfide)/r-Graphene Oxide/Chitosan as a novel potential candidate for tissue engineering. Int J Biol Macromol 154:18–24
Khorasani MM, Saeb MR, Mohammadi Y, Ahmadi M (2014) The evolutionary development of chain microstructure during tandem polymerization of ethylene: a Monte Carlo simulation study. Chem Eng Sci 111:211–219
Kozera-Sucharda B, Gworek B, Kondzielski I (2020) The simultaneous removal of zinc and cadmium from multicomponent aqueous solutions by their sorption onto selected natural and synthetic zeolites. Minerals 10:343
Krishna R, van Baten J (2008) Separating n-alkane mixtures by exploiting differences in the adsorption capacity within cages of CHA, AFX and ERI zeolites. Sep Purif Technol 60:315–320
Król M (2020) Natural vs. synthetic zeolites. Crystals 10:622
Kumar P, Sung C-Y, Muraza O, Cococcioni M, Al Hashimi S, McCormick A et al (2011) H2S adsorption by Ag and Cu ion exchanged faujasites. Microporous Mesoporous Mater 146:127–133
Laaksonen A, Tu Y (1999) Chapter 1—Methods of incorporating quantum mechanical calculations into molecular dynamics simulations. In: Theoretical and Computational Chemistry, Balbuena PB, Seminario JM (Eds), Elsevier, Vol. 7, pp. 1–29.
Lachet V, Boutin A, Tavitian B, Fuchs AH (1998) Computational study of p-xylene/m-xylene mixtures adsorbed in NaY zeolite. J Phys Chem B 102:9224–9233
Lachet V, Buttefey S, Boutin A, Fuchs AH (2001) Molecular simulation of adsorption equilibria of xylene isomer mixtures in faujasite zeolites. A study of the cation exchange effect on adsorption selectivity. Phys Chem Chem Phys 3:80–6
Lima EC, Sher F, Guleria A, Saeb MR, Anastopoulos I, Tran HN et al (2021) Is one performing the treatment data of adsorption kinetics correctly? J Environ Chem Eng 9:104813
Liu D, Wu Y, Xia Q, Li Z, Xi H (2013) Experimental and molecular simulation studies of CO 2 adsorption on zeolitic imidazolate frameworks: ZIF-8 and amine-modified ZIF-8. Adsorption 19:25–37
Liu S, Yang X (2006) Gibbs ensemble Monte Carlo simulation of supercritical CO 2 adsorption on Na A and Na X zeolites. J Chem Phys 124:244705
Loewenstein W (1954) The distribution of aluminum in the tetrahedra of silicates and aluminates. Am Mineralogist: J Earth Planetary Mater 39:92–96
Mahmodi G, Dangwal S, Zarrintaj P, Zhu M, Mao Y, McLlroy DN et al (2020) NaA zeolite-coated meshes with tunable hydrophilicity for oil-water separation. Sep Purif Technol 240:116630
Mahmodi G, Zarrintaj P, Taghizadeh A, Taghizadeh M, Manouchehri S, Dangwal S et al (2020) From microporous to mesoporous mineral frameworks: an alliance between zeolite and chitosan. Carbohydr Res 489:107930
Maroo SC, Chung J (2008) Molecular dynamic simulation of platinum heater and associated nano-scale liquid argon film evaporation and colloidal adsorption characteristics. J Colloid Interface Sci 328:134–146
Mashhadzadeh AH, Vahedi AM, Ardjmand M, Ahangari MG (2016) Investigation of heavy metal atoms adsorption onto graphene and graphdiyne surface: a density functional theory study. Superlattices Microstruct 100:1094–1102
McQuarrie D (2000) Statistical mechanics university science books. CA, Sausalito, pp 222–3
Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E (1953) Equation of state calculations by fast computing machines. J Chem Phys 21:1087–1092
Mintova S, Gilson J-P, Valtchev V (2013) Advances in nanosized zeolites. Nanoscale 5:6693–6703
Mohammadi Y, Saeb MR, Penlidis A, Jabbari E, Zinck P, Stadler FJ et al (2018) Intelligent monte carlo: a new paradigm for inverse polymerization engineering. Macromol Theory Simul 27:1700106
Nagar H, Badhrachalam N, Rao VB, Sridhar S (2019) A novel microbial fuel cell incorporated with polyvinylchloride/4A zeolite composite membrane for kitchen wastewater reclamation and power generation. Mater Chem Phys 224:175–185
Nakatsuji H, Nakashima H, Kurokawa Y, Ishikawa A (2007) Solving the Schrödinger equation of atoms and molecules without analytical integration based on the free iterative-complement-interaction wave function. Phys Rev Lett 99:240402
Narasimhan L, Boulet P, Kuchta B, Schaef O, Denoyel R, Brunet P (2009) Molecular simulations of water and paracresol in MFI zeolite-a Monte Carlo study. Langmuir 25:11598–11607
Newsome D, Gunawan S, Baron G, Denayer J, Coppens M-O (2014) Adsorption of CO 2 and N 2 in Na–ZSM-5: effects of Na+ and Al content studied by Grand Canonical Monte Carlo simulations and experiments. Adsorption 20:157–171
Nguyen CM, Reyniers M-F, Marin GB (2010) Theoretical study of the adsorption of C1–C4 primary alcohols in H-ZSM-5. Phys Chem Chem Phys 12:9481–9493
Nizami AS, Ouda OKM, Rehan M, El-Maghraby AMO, Gardy J, Hassanpour A et al (2016) The potential of Saudi Arabian natural zeolites in energy recovery technologies. Energy 108:162–171
O’Malley AJ, Catlow CRA (2013) Molecular dynamics simulations of longer n-alkanes in silicalite: a comparison of framework and hydrocarbon models. Phys Chem Chem Phys 15:19024–19030
Papadopoulou A, Becker ED, Lupkowski M, van Swol F (1993) Molecular dynamics and Monte Carlo simulations in the grand canonical ensemble: local versus global control. J Chem Phys 98:4897–4908
Pascual P, Ungerer P, Tavitian B, Pernot P, Boutin A (2003) Development of a transferable guest–host force field for adsorption of hydrocarbons in zeolites I. Reinvestigation of alkane adsorption in silicalite by grand canonical Monte Carlo simulation. Phys Chem Chem Phys 5:3684–93
Phan A, Doonan CJ, Uribe-Romo FJ, Knobler CB, O’Keeffe M, Yaghi OM (2010) Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc Chem Res 43:58–67
Pillai RS, Sebastian J, Jasra RV (2012) Grand canonical Monte Carlo simulation and volumetric equilibrium studies for adsorption of nitrogen, oxygen, and argon in cadmium (II) exchanged zeolite A. J Porous Mater 19:683–693
Prakash M, Sakhavand N, Shahsavari R (2013) H2, N2, and CH4 gas adsorption in zeolitic imidazolate framework-95 and-100: Ab initio based grand canonical Monte Carlo simulations. J Phys Chem C 117:24407–24416
Prasanth K, Pillai RS, Peter SA, Bajaj H, Jasra R, Chung H et al (2008) Hydrogen uptake in palladium and ruthenium exchanged zeolite X. J Alloy Compd 466:439–446
Rahmati M, Mills D, Urbanska A, Saeb M, Venugopal J, Ramakrishna S et al (2020) Electrospinning for tissue engineering applications. Prog Mater Sci 117:100721
Rahmati M, Modarress H (2009a) Nitrogen adsorption on nanoporous zeolites studied by Grand Canonical Monte Carlo simulation. J Mol Struct (thoechem) 901:110–116
Rahmati M, Modarress H (2009b) Grand canonical Monte Carlo simulation of isotherm for hydrogen adsorption on nanoporous siliceous zeolites at room temperature. Appl Surf Sci 255:4773–4778
Rassoulinejad-Mousavi SM, Azamat J, Khataee A, Zhang Y (2020) Molecular dynamics simulation of water purification using zeolite MFI nanosheets. Sep Purif Technol 234:116080
Razmus DM, Hall CK (1991) Prediction of gas adsorption in 5A zeolites using Monte Carlo simulation. AIChE J 37:769–779
Saeb MR, Mohammadi Y, Ahmadi M, Khorasani MM, Stadler FJ (2015) A Monte Carlo-based feeding policy for tailoring microstructure of copolymer chains: reconsidering the conventional metallocene catalyzed polymerization of α-olefins. Chem Eng J 274:169–180
Saeb MR, Mohammadi Y, Pakdel AS, Penlidis A (2016) Molecular architecture manipulation in free radical copolymerization: an advanced monte carlo approach to screening copolymer chains with various comonomer sequence arrangements. Macromol Theory Simul 25:369–382
Salmankhani A, Karami Z, Mashhadzadeh AH, Ganjali MR, Vatanpour V, Esmaeili A, et al. (2020) New insights into H2S adsorption on graphene and graphene-like structures: a comparative DFT study. C 6:74
Sargazi G, Afzali D, Mostafavi A, Shadman A, Rezaee B, Zarrintaj P et al (2019) Chitosan/polyvinyl alcohol nanofibrous membranes: towards green super-adsorbents for toxic gases. Heliyon 5:e01527
Servatan M, Ghadiri M, Damanabi AT, Bahadori F, Zarrintaj P, Ahmadi Z et al (2018) Zeolite-based catalysts for exergy efficiency enhancement: the insights gained from nanotechnology. Mater Today Proc 5:15868–15876
Servatan M, Zarrintaj P, Mahmodi G, Kim S-J, Ganjali MR, Saeb MR et al (2020b) Zeolites in drug delivery: progress, challenges and opportunities. Drug Discov Today 25:642–656
Servatan M, Ghadiri M, Yazdi MK, Jouyandeh M, Mahmodi G, Samadi A et al (2020) Synthesis of cost-effective hierarchical MFI-type mesoporous zeolite: introducing diatomite as silica source. Silicon. https://doi.org/10.1007/s12633-020-00786-7
Shah MS, Tsapatsis M, Siepmann JI (2015) Monte Carlo simulations probing the adsorptive separation of hydrogen sulfide/methane mixtures using all-silica zeolites. Langmuir 31:12268–12278
Shahmoradi A, Ghorbanzadeh Ahangari M, Jahanshahi M, Hamed Mashhadzadeh A (2020a) Adsorption of hazardous atoms on the surface of TON zeolite and bilayer silica: a DFT study. J Mol Model 26:119
Shahmoradi A, Ghorbanzadeh Ahangari M, Jahanshahi M, Mirghoreishi M, Fathi E, Hamed Mashhadzadeh A (2020b) Removal of methylmercaptan pollution using Ni and Pt-decorated graphene: an ab-initio DFT study. J Sulfur Chem 41:593–604
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM (2010) Science and technology for water purification in the coming decades. In: Nanoscience and technology: a collection of reviews from nature Journals, World Scientific pp. 337–46
Slaughter M, Yu J-Y (1991) Partial binding energies and structural properties of the heulandite/clinoptilolite series. In: Clay Minerals Society 28th Annual Meeting, Vol. 773, p. 145
Smit B, Krishna R (2001) Monte Carlo simulations in zeolites. Curr Opin Solid State Mater Sci 5:455–461
Song MK, No KT (2007) Molecular simulation of hydrogen adsorption in organic zeolite. Catal Today 120:374–382
Sweatman M, Quirke N (2001) Modelling gas adsorption in slit-pores using Monte Carlo simulation. Mol Simul 27:295–321
Thomas AM, Subramanian Y (2017) Hexane isomers in faujasite: anomalous diffusion and kinetic separation. J Phys Chem C 121:14745–14756
Thomas AM, Subramanian Y (2019) Bridging the gap between diffusivities from experiment and molecular dynamics: n-hexane and 2, 2-dimethyl butane in zeolite BEA. Microporous Mesoporous Mater 287:124–134
Timón V, Senent ML, Hochlaf M (2015) Structural single and multiple molecular adsorption of CO2 and H2O in zeolitic imidazolate framework (ZIF) crystals. Microporous Mesoporous Mater 218:33–41
Treybal RE (1980) Mass transfer operations. New York, p. 466
Turgman-Cohen S, Araque JC, Hoek EM, Escobedo FA (2013) Molecular dynamics of equilibrium and pressure-driven transport properties of water through LTA-type zeolites. Langmuir 29:12389–12399
Ungerer P, Tavitian B, Boutin A (2005) Applications of molecular simulation in the oil and gas industry: Monte Carlo methods, Editions Technip.
Valencia-Ortega M, Fuentes-Azcatl R, Dominguez H (2019) Carbon dioxide adsorption on a modified zeolite with sodium dodecyl sulfate surfactants: a molecular dynamics study. J Mol Graph Model 92:243–248
Van Tassel P, Davis H, McCormick A (1991) Monte Carlo calculations of adsorbate placement and thermodynamics in a micropore: Xe in NaA. Mol Phys 73:1107–1125
Vatanpour V, Khadem SSM, Dehqan A, Al-Naqshabandi MA, Ganjali MR, Hassani SS et al (2021) Efficient removal of dyes and proteins by nitrogen-doped porous graphene blended polyethersulfone nanocomposite membranes. Chemosphere 263:127892
Vinaches P, Bernardo-Gusmão K, Pergher SB (2017) An introduction to zeolite synthesis using imidazolium-based cations as organic structure-directing agents. Molecules 22:1307
Wajima T, Haga M, Kuzawa K, Ishimoto H, Tamada O, Ito K et al. (2006) Zeolite synthesis from paper sludge ash at low temperature (90Eæ C) with addition of diatomite 132: 244–52
Wang Y, Jia H, Chen P, Fang X, Du T (2020) Synthesis of La and Ce modified X zeolite from rice husk ash for carbon dioxide capture. J Market Res 9:4368–4378
Weitkamp J (2000) Zeolites and Catalysis. Solid State Ionics 131:175–188
Wu C, Buyya R (2015) Chapter 18—real option theory and monte carlo simulation. In: Wu C, Buyya R (eds) Cloud data centers and cost modeling. Morgan Kaufmann, Elsevier, pp 707–72
Yazdi MK, Zarrintaj P, Hosseiniamoli H, Mashhadzadeh AH, Saeb MR, Ramsey JD et al (2020) Zeolites for theranostic applications. J Mater Chem B 8:5992–6012
Zarrintaj P, Mahmodi G, Manouchehri S, Mashhadzadeh AH, Khodadadi M, Servatan M et al (2020) Zeolite in tissue engineering: Opportunities and challenges. MedComm 1:5–34
Zhang J, Burke N, Zhang S, Liu K, Pervukhina M (2014) Thermodynamic analysis of molecular simulations of CO2 and CH4 adsorption in FAU zeolites. Chem Eng Sci 113:54–61
Acknowledgements
The authors thank CAPES, FAPERGS, and CNPq for funds. They also thank Dr. Bruno Azambre from Laboratoire de Chimie et Physique-Approche Multi-Echelle des Milieux Complexes, Université de Lorraine, for useful comments and discussions.
Funding
N/A
Author information
Authors and Affiliations
Contributions
AS wrote the original draft of article, SSMK wrote the original draft of article, FS generated formal analysis, AHM did conceptualization, PZ generated formal analysis, SH generated formal analysis, AM generated formal analysis, NR generated formal analysis, ECL, MS, and RSV were involved in writing, reviewing, and editing to give the review its final form, , , MRS helped in visualization and final checking of the whole manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salmankhani, A., Mousavi Khadem, S.S., Seidi, F. et al. Adsorption onto zeolites: molecular perspective. Chem. Pap. 75, 6217–6239 (2021). https://doi.org/10.1007/s11696-021-01817-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01817-2