Abstract
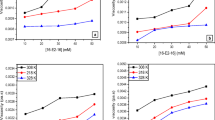
Surfactants and polymers are widely used chemicals in different applied fields like pharmaceutical, textile, food, and cosmetic industries. Therefore, the study of surfactant–polymer interaction can improve their activity in related applied fields. Herein, we studied the interaction of polymer, sodium carboxymethyl cellulose (SCMC) with two surfactants. The interaction of SCMC with cetyltrimethylammonium bromide (CTAB), a cationic surfactant, was assessed through conductivity measurement technique, while the interaction between non-ionic surfactant, Triton X-100 (TX-100) and SCMC was assessed by cloud point (CP) analysis. The effect of different organic additives (glucose, maltose, and urea) on the micellar behavior of the CTAB + SCMC mixture and phase partitioning behavior of TX-100 + SCMC mixture was investigated. The critical micelle concentration (cmc) of the CTAB + SCMC system having 0.3 mmolkg−1 SCMC was augmented linearly as a function of temperature and concentration of organic additives. The cmc values of CTAB + SCMC system increased from 1.28 to 1.44 mmolkg−1 and 1.18 to 1.33 mmolkg−1 with the increase in concentration from 0.1 to 8.0 mmolkg−1 of glucose and maltose, respectively. The extent of counterion binding (β) was obtained to be temperature-dependent. The CP values of TX-100 + SCMC were experienced to be reduced up to a certain concentration of glucose/maltose and then augment with enhancing the concentration of glucose and maltose, while the CP values increased monotonically with the concentration of urea. The Gibbs free energy values indicate the spontaneous micellization of the CTAB + SCMC system and non-spontaneous phase partitioning of the TX-100 + SCMC system. The enthalpy and entropy change as well as their compensation were studied and explained with proper explanation.



Similar content being viewed by others
References
Abedin MJ, Mahbub S, Rahman MM, Hoque MA, Kumar D, Khan JM, El-Sherbeeny AM (2021) Interaction of tetradecyltrimethylammonium bromide with bovine serum albumin in different compositions Effect of temperatures and electrolytes/urea. Chinese J Chem Eng 29:279–287
Acosta E, Bisceglia M, Kurlat DH (2005) Self-aggregation in aqueous TRITON X-100 solutions near CMC. Phys Chem Liq 43:269–275
Aferni AEL, Guettari M, Tajouri T (2016) Effect of polymer conformation on polymer-surfactant interaction in salt-free water. Coll Polym Sci 294:1097–1106
Afonin KA, Bindewald E, Yaghoubian AJ, Voss N, Jacovetty E, Shapiro BA, Jaeger L (2010) In vitro assembly of cubic RNA-based scafolds designed in silico. Nat Nanotechnol 5:676–682
Akbaş H, Kartal Ç (2006) Conductometric studies of hexadecyltrimethylammonium bromide in aqueous solutions of ethanol and ethylene glycol. Coll J 68:125–130
Aktar S, Molla MR, Mahbub S, Rub MA, Hoque MA, Islam DMS (2018) Effect of temperature and salt/alcohol on the interaction of tetradecyltrimethylammonium bromide/Triton X-100 with moxifloxacin hydrochloride: a multitechnique approach. J Dispers Sci Technol 40:574–586
Aktar S, Saha M, Mahbub S, Halim MA, Rub MA, Hoque MA, Islam DMS, Kumar D, Alghamdi YG, Asiri AM (2020) Influence of polyethylene glycol on the aggregation/clouding phenomena of cationic and non-ionic surfactants in attendance of electrolytes (NaCl & Na2SO4): an experimental and theoretical analysis. J Mol Liq 306:112880
Attwood D, Florence AT (1983) Surfactant systems their chemistry pharmacy and biological properties. Chapman and Hall, New York
Azum N, Rub MA, Asiri AM (2018) Interaction of antipsychotic drug with novel surfactants: micellization and binding studies. Chin J Chem Eng 26:566–573
Banipal TS, Kaur H, Banipal PK, Sood AK (2014) Effect of head groups, temperature, and polymer concentration on surfactant-polymer interactions. J Surfact Deterg 14:1181–1191
Batigöc C, Akbas H, Boz M (2011) Thermodynamics of non-ionic surfactant Triton X-100-cationic surfactant mixtures at the cloud point. J Chem Thermodyn 43:1800–1803
Bhardwaj V, Bhardwaj T, Sharma K, Gupta A, Chauhan S, Cameotra SS, Sharma S, Gupta R, Sharma P (2014) Drug-surfactant interaction: thermo-acoustic investigation of sodium dodecyl sulfate and antimicrobial drug (levofloxacin) for potential pharmaceutical application. RSC Adv 4:24935–24943
Bhardwaj V, Sharma P, Chauhan MS, Chauhan S (2016) Micellization, interaction and thermodynamic study of butylated hydroxyanisole (synthetic antioxidant) and sodium dodecyl sulfate in aqueous-ethanol solution at 25, 30 and 35 °C. J Saudi Chem Soc 20:S109–S114
Chakraborty A, Sarkar M, Basak S (2005) Stabilizing effect of low concentrations of urea on reverse micelles. J Coll Interf Sci 287:312–317
Chakraborty T, Chakraborty I, Ghosh S (2006) Sodium carboxymethylcellulose−CTAB interaction: a detailed thermodynamic study of polymer−surfactant interaction with opposite charges. Langmuir 22:9905–9913
Chauhan S, Sharma V, Singh K, Chauhan MS (2018) Effect of maltodextrin and temperature on micellar behavior of bile salts in aqueous medium: conductometric and spectrofluorimetric studies. Z Phys Chem 233:1091–1108
Das S, Naskar B, Ghosh S (2014) Influence of temperature and organic solvents (isopropanol and 1,4-dioxane) on the micellization of cationic gemini surfactant (14-4-14). Soft Matter 10:2863–2875
Dey A, Patra N, Mal A, Ghosh S (2017) Impact of organic polar solvents (DMSO and DMF) on the micellization and related behavior of an anionic (AOT), cationic (CEM2AB) and cationic gemini surfactant (16-5-16). J Mol Liq 244:85–96
Douglas SM, Dietz H, Liedl T, Högberg B, Graf F, Shih WM (2009) Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 459:414–418
Enea O, Jolicoeur C (1982) Heat capacities and volumes of several oligopeptides in urea-water mixtures at 25degreeC Some implications for protein unfolding. J Phys Chem 86:3870–3881
Han SK, Lee SM, Schott H (1988) The effect of protein denaturants on the cloud point of a nonionic surfactant. J Coll Interf Sci 126:393–396
Hansson P, Lindman B (1996) Surfactant-polymer interactions. Curr Opin Coll Interf Sci 1:604–613
Hasan MZ, Mahbub S, Hoque MA, Rub MA, Kumar D (2020) Investigation of mixed micellization study of sodium dodecyl sulfate and tetradecyltrimethylammonium bromide mixtures at different compositions: effect of electrolytes and temperatures. J Phys Org Chem 33:e4047
He Y, Ye T, Su M, Zhang C, Ribbe AE, Jiang W, Mao C (2008) Hierarchical self-assembly of DNA into symmetric supramolecular polyhedral. Nature 452:198–201
Hoque MA, Ahmed F, Halim MA, Molla MR, Rana S, Rahman MA, Rub MA (2018a) Influence of salt, temperature on the interaction of bovine serum albumin with cetylpyridinium chloride: insights from experimental and molecular dynamics simulation. J Mol Liq 260:121–130
Hoque MA, Alam MM, Molla MR, Rana S, Rub MA, Halim MA, Khan MA, Akhtar F (2018b) Interaction of cetyltrimethylammonium bromide with drug in aqueous/electrolyte solution: a conductometric and molecular dynamics method study. Chin J Chem Eng 26:159–167
Hsiao CC, Wang TY, Tsao HK (2005) Counterion condensation and release in micellar solutions. J Chem Phys 122:144702
Iinuma R, Ke Y, Jungmann R, Schlichthaerle T, Woehrstein JB, Yin P (2014) Polyhedra self-assembled from DNA tripods and characterized with 3D DNA-PAINT. Science 344:65–69
Ito E, Yip KW, Katz D, Fonseca SB, Hedley DW, Chow S, Xu GW, Wood TE, Bastianutto C, Schimmer AD, Kelley SO, Liu FF (2009) Potential use of cetrimonium bromide as an apoptosis-promoting anticancer agent for head and neck cancer. Mol Pharmacol 76:969–983
Jia X, Bo H, He Y (2019) Synthesis and characterization of a novel surfactant used for aqueous film-forming foam extinguishing agent. Chem Paper 73:1777–1784
Khan N, Brettmann B (2019) Intermolecular interactions in polyelectrolyte and surfactant complexes in solution. Polymers 11:51–56
King NP, Sheffler W, Sawaya MR, Vollmar BS, Sumida JP, André I, Gonen T, Yeates TO, Baker D (2012) Computational design of self-assembling protein nanomaterials with atomic level accuracy. Science 336:1171–1174
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359:710–712
Kumar D, Rub MA (2016) Aggregation behavior of amphiphilic drug promazine hydrochloride and sodium dodecylbenzenesulfonate mixtures under the influence of NaCl/urea at various concentration and temperatures. J Phys Org Chem 29:394–405
Kumar D, Hidayathulla S, Rub MA (2018) Association behavior of a mixed system of the antidepressant drug imipramine hydrochloride and dioctyl sulfosuccinate sodium salt: Effect of temperature and salt. J Mol Liq 271:254–264
Kumar D, Rub MA (2019) Role of cetyltrimethylammonium bromide (CTAB) surfactant micelles on kinetics of [Zn(II)-Gly-Leu]+ and ninhydrin. J Mol Liq 274:639–645
Kumar D, Rub MA (2020a) Influence of dicationic quaternary ammonium gemini surfactant system on metal-amino acid complex-ninhydrin reaction. Mater Chem Phys 248:122926
Kumar D, Rub MA (2020b) Alkanediyl-α, ω-type gemini micelles–catalyzed study between ninhydrin and [Ni(II)-Trp]+ complex. Coll Polym Sci 298:1411–1421
Kumar N, Tyagi R (2015) Analysis of the interactions of polyvinylpyrrolidone with conventional anionic and dimeric anionic surfactant. J Dispers Sci Technol 36:1601–1606
Kumar S, Parikh K (2012) Influence of temperature and salt on association and thermodynamic parameters of micellization of a cationic gemini surfactant. J Appl Sol Chem Model 1:65–73
Lerbret A, Bordat P, Affouard F, Descamps M, Migliardo F (2005) How homogeneous are the Trehalose, Maltose, and Sucrose water solution? An insight from molecular dynamics simulation. J Phys Chem B 109:11046–11057
Li Y, Ghoreishi SM, Warr J, Bloor DM, Holzwarth JF, Wyn-Jones E (1999) Interactions between a nonionic copolymer containing different amounts of covalently bonded vinyl acrylic acid and surfactants: EMF and microcalorimetry studies. Langmuir 15:6326–6332
Lumry R, Rajender S (1970) Enthalpy-entropy compensation phenomena in water solutions of proteins and small molecules: a ubiquitous properly of water. Biopolymers 9:1125–1127
Ma K, Gong Y, Aubert T, Turker MZ, Kao T, Doerschuk PC, Wiesner U (2018) Self-assembly of highly symmetrical, ultrasmall inorganic cages directed by surfactant micelles. Nature 558:577–580
Mahajan S, Shaheen A, Banipal TS, Mahajan RK (2010) Cloud point and surface tension studies of triblock copolymer-ionic surfactant mixed systems in the presence of amino acids or dipeptides and electrolytes. J Chem Eng Data 55:3995–4001
Mahbub S (2020) The impact of electrolyte and urea on the phase separation of Triton X-100. J Mol Liq 307:112912
Mahbub S, Shahriar I, Iqfath M, Rub MA, Hoque MA, Halim MA, Khan MA, Asiri AM (2019a) Influence of alcohols/electrolytes on the interaction of reactive red dye with surfactant and removal of dye from solutions. J Environ Chem Eng 7:103364
Mahbub S, Rub MA, Hoque MA, Khan MA (2019b) Influence of NaCl/urea on the aggregation behavior of dodecyltrimethylammonium chloride and sodium dodecyl sulfate at varying temperatures and compositions: Experimental and theoretical approach. J Phys Org Chem 32:e3917
Mahbub S, Rub MA, Hoque MA, Khan MA, Kumar D (2019c) Micellization behavior of cationic and anionic surfactant mixtures at different temperatures: effect of sodium carbonate and sodium phosphate salts. J Phys Org Chem 32:e3967
Mahbub S, Akter P, Rub HMA, MA, (2019d) Conductometric study of influence of urea on interactions of sodium dodecyl sulfate with cefradine. Russian J Phy Chem A 93:2494–2501
Mahbub S, Akter S, Luthfunnessa AP, Hoque MA, Rub MA, Kumar D, Alghamdi YG, Asiri AM, Džudžević-Čančar H (2020) Effects of temperature and polyols on the ciprofloxacin hydrochloride-mediated micellization of sodium dodecyl sulfate. RSC Adv 10:14531–14541
Mestri RS, Pratap AP, Panchal KH, Gamot K, Datir KA (2020) Synthesis of cleavable silicone surfactant for water-repellent application. Chem Paper 74:1407–1416
Minatti E, Zanette D (1996) Salt effects on the interaction of poly(ethylene oxide) and sodium dodecyl sulfate measured by conductivity. Coll Surf A 113:237–246
Olad A, Ilghami F, Nosrati R (2012) Surfactant-assisted synthesis of polyaniline nanofibres without shaking and stirring: effect of conditions on morphology and conductivity. Chem Paper 66:757–764
Patidar P, Bahadur A (2018) Modulating effect of different biomolecules and other additives on cloud point and aggregation of amphiphilic linear and starblock copolymer. J Mol Liq 249:219–226
Patra N, Mal A, Dey A, Ghos S (2019) Influence of solvent, electrolytes, β-CD, OTAB on the krafft temperature and aggregation of sodium tetradecyl sulfate. J Mol Liq 280:307–313
Ahsan SMA, Mahbub S, Hoque MA, Khan MA, Kumar D, Khan JM, El-Sherbeeny AM (2020) Influence of electrolytes on the cloud point phenomena of tween-80+lomefloxacin hydrochloride mixtures and their thermodynamic parameters. J Mol Liq 318:113999
Rahman M, Hoque MA, Rub MA, Khan MA (2019) Interaction of cetyltrimethylammonium bromide with cefixime trihydrate drug at different temperatures and compositions: Effect of different electrolytes. Chin J Chem Eng 27:1895–1903
Robinson DR, Jencks WP (1965) The Effect of compounds of the urea-guanidinium class on the activity coefficient of acetyltetraglycine ethyl ester and related compounds. J Am Chem Soc 87:2462–2470
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. John Wiley & Sons, New York
Rub MA, Azum N, Khan F, Asiri AM (2017) Surface, micellar, and thermodynamic properties of antidepressant drug nortriptyline hydrochloride with TX-114 in aqueous/urea solutions. J Phys Org Chem 30:e3676
Sandomierski M, Poniedziałek K, Bielicka-Daszkiewicz K, Voelkel A (2020) Influence of diazonium and surfactant modification of the mesoporous material on its adsorption properties. Chem Paper 74:929–938
Sergey AK, Volodymyr OD, Natalia AG (2010) Phase separation in non-ionic surfactant Triton X-100 solutions in the presence of phenol. Chem Paper 64:91–97
Sharma KS, Patil SR, Rakshit AK (2003) Study of the cloud point of C12En nonionic surfactants: effect of additives. Coll Surf A 219:67–74
Siddiqui H, Kamil M, Panda M, Kabir-ud-Din, (2014) Solubilization of phenanthrene and fluorene in equimolar binary mixtures of gemini/conventional surfactants. Chin J Chem Eng 22:1009–1015
Shi W, Li M, Huang X, Ren H, Guo F, Tang Y, Lu C (2020) Construction of CuBi2O4/Bi2MoO6 p-n heterojunction with nanosheets-onmicrorods structure for improved photocatalytic activity towards broadspectrum antibiotics degradation. Chem Eng J 394:125009
Wang S, Zhao L, Huang W, Zhao H, Chen J, Cai Q, Jiang X, Lu C, Shi W (2021) Solvothermal synthesis of CoO/BiVO4 p-n heterojunction with micro-nano spherical structure for enhanced visible light photocatalytic activity towards degradation of tetracycline. Mater Res Bull 135:111161
Wang X, Wang J, Wang Y, Ye J, Yan H, Thomas RK (2005) Properties of mixed micelles of cationic gemini surfactants and nonionic surfactant triton X-100: effects of the surfactant composition and the spacer length. J Coll Interf Sci 286:739–746
Wetlaufer DB, Malik SK, Stoller L, Coffin RL (1964) Nonpolar group participation in the denaturation of proteins by urea and guanidinium salts model compound studies. J Am Chem Soc 86:508–514
Yoon G (2011) Dielectric properties of glucose in bulk aqueous solutions: influence of electrode polarization and modeling. Biosens Bioelectron 26:2347–2353
Zhang S, Yu J, Wu J, Tong W, Lei Q, Fang W (2014) Micellization parameters of six gemini quaternary ammonium surfactants from measurements of conductivity and surface tension. J Chem Eng Data 59:2891–2900
Zhou Z, Zhu S, Gong J, Zhu M, Luo W (2018) Experimental study on methane solubilization by organic surfactant aggregates. Chem Paper 72:1467–1475
Zhu Q, Sun Y, Na F, Wei J, Xu S, Lia Y, Guo F (2019) Fabrication of CdS/titanium-oxo-cluster nanocomposites based on a Ti32 framework with enhanced photocatalytic activity for tetracycline hydrochloride degradation under visible light. Appl Catal B 254:541–550
Zhu Q, Sun Y, Xu S, Li Y, Lin X, Qin Y (2020) Rational design of 3D/2D In2O3 nanocube/ZnIn2S4 nanosheet heterojunction photocatalyst with large-area “high-speed channels” for photocatalytic oxidation of 2,4-dichlorophenol under visible light. J Hazard Mater 382:121098
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alam, M.M., Mahbub, S., Hosen, M.M. et al. A conductivity and cloud point investigation of interaction of cationic and non-ionic surfactants with sodium carboxymethyl cellulose: effect of polyols and urea. Chem. Pap. 75, 3457–3468 (2021). https://doi.org/10.1007/s11696-021-01568-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01568-0