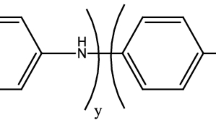
Abstract
Electroactive polymers such as polyaniline and its derivatives have many potential applications, but the nature of the dopants can have an adverse effect on their properties. Polyaniline (PANI), poly(o-methoxyaniline) (POMA) and poly(o-toluidine) (POT) have been synthesised using appropriate organic acid dopants (2-naphthalenesulfonic acid and 1,5-naphthalenedisulfonic acid) via electrochemical and chemical polymerisation methods, where the later was achieved at pH (1–2) in the presence of ammonium persulfate as an oxidant. The effects of the monomers and organic dopants on the physiochemical properties and morphological surface of the polymers were studied by means of Fourier transform infrared spectroscopy, electrical conductivity measurements, thermogravimetric analysis and scanning electron microscopy (SEM). The above films were also synthesised electrochemically whilst varying conditions such as scan rate, scan number and in aqueous solution of organic acids using cyclic voltammetry techniques. Cyclic voltammetry experiments have been employed to analyse the growth behaviour and electrochemical stability of films in the background electrolytes (monomer-free). It was found that PANI salts displayed higher doping levels and conductivity while POMA and POT salts were found to show lower conductivity and doping levels, respectively. Furthermore the electrochemical response of films was different in the stability and this may be because of the methoxy (–OCH3) and methyl (–CH3) groups located at the ortho position of the aromatic ring structure of the monomers (o-methoxyaniline and o-toluidine). The –OCH3 and–CH3 groups have significant steric effects and can reduce to form long chains of polymers and also decrease conductivity. The SEM showed that the morphologies of the prepared conducting polymers were different, which was associated with the nature and size of the dopant and monomer present in the bath solution.








Similar content being viewed by others
References
Abdiryim T, Xiao-Gang Z, Jamal R (2005) Synthesis and characterization of poly (o-toluidine) doped with organic sulfonic acid by solid-state polymerization. J Appl Polym Sci 96:1630–1634. https://doi.org/10.1002/app.21614
Alabdullah SS, Ismail HK, Ryder KS, Abbott AP (2020) Evidence supporting an emulsion polymerisation mechanism for the formation of polyaniline. Electrochim Acta 354:136737. https://doi.org/10.1016/j.electacta.2020.136737
Alesary HF, Ismail K, Khudhair AF, Mohammed MQ (2018) Effects of dopant ions on the properties of polyaniline conducting polymer. Orient J Chem 34:2525. https://doi.org/10.13005/ojc/340539
Aphesteguy JC, Jacobo SE (2007) Synthesis of a soluble polyaniline–ferrite composite: magnetic and electric properties. J Mater Sci 42:7062–7068. https://doi.org/10.1007/s10853-006-1423-7
Banaszczyk J, Schwarz A, De Mey G, Van Langenhove L (2010) The Van der Pauw method for sheet resistance measurements of polypyrrole-coated para-aramide woven fabrics. J Appl Polym Sci 117:2553–2558. https://doi.org/10.1002/app.32186
Bauermann L, Bartlett P (2005) EQCM measurements of the ion and solvent flux in thin poly (aniline)–poly (styrenesulfonate) films during redox switching. Electrochim Acta 50:1537–1546. https://doi.org/10.1016/j.electacta.2004.10.011
Bazzaoui EA, Aeiyach S, Lacaze P (1994) Low potential electropolymerization of thiophene in aqueous perchloric acid. J Electroanal Chem 364:63–69. https://doi.org/10.1016/0022-0728(93)02910-A
Borole D, Kapadi U, Kumbhar P, Hundiwale D (2002) Influence of inorganic and organic supporting electrolytes on the electrochemical synthesis of polyaniline, poly (o-toluidine) and their copolymer thin films. Mater Lett 56:685–691. https://doi.org/10.1016/S0167-577X(02)00579-7
Borole D, Kapadi U, Mahulikar P, Hundiwale D (2004) Electrochemical behaviour of polyaniline, poly (o-toluidine) and their copolymer in organic sulphonic acids. Mater Lett 58:3816–3822. https://doi.org/10.1016/j.matlet.2004.07.035
Borole D, Kapadi U, Mahulikar P, Hundiwale D (2006) Electrochemical synthesis and characterization of conducting copolymer: poly (o-aniline-co-o-toluidine). Mater Lett 60:2447–2452. https://doi.org/10.1016/j.matlet.2006.01.014
Cao Y, Andreatta A, Heeger AJ, Smith P (1989) Influence of chemical polymerization conditions on the properties of polyaniline. Polymer 30:2305–2311. https://doi.org/10.1016/0032-3861(89)90266-8
Catedral M, Tapia A, Sarmago R, Tamayo J, Del Rosario E (2004) Effect of dopant ions on the electrical conductivity and microstructure of polyaniline (emeraldine salt). Science Diliman 16:41–46
Christinelli W, da Trindade L, Trench A, Quintans C, Paranhos C, Pereira E (2017) High-performance energy storage of poly (o-methoxyaniline) film using an ionic liquid as electrolyte. Energy 141:1829–1835. https://doi.org/10.1016/j.energy.2017.11.026
Chujo Y (2011) Conjugated polymer synthesis: methods and reactions. Wiley, New Jersey
Delvaux M, Duchet J, Stavaux PY, Legras R, Demoustier-Champagne S (2000) Chemical and electrochemical synthesis of polyaniline micro-and nano-tubules. Synth Met 113:275–280. https://doi.org/10.1016/S0379-6779(00)00226-5
Deshpande PP, Murali M, Deshpande PP, Galphade VS, More MA (2013) Conducting poly (o-anisidine)-coated steel electrodes for supercapacitors. Chem Pap 67:1066–1071. https://doi.org/10.2478/s11696-013-0317-9
Elmansouri A et al (2007) Spectroscopic characterization of electrodeposited poly (o-toluidine) thin films and electrical properties of ITO/poly (o-toluidine)/aluminum Schottky diodes. Act Passive Electron Compon. https://doi.org/10.1155/2007/17846
Gruia VT, Ispas A, Efimov I, Bund A (2020) Cation exchange behavior during the redox switching of poly (3, 4-ethylenedioxythiophene) films. J Solid State Electrochem 24:3231–3244. https://doi.org/10.1007/s10008-020-04809-6
Gustafson MP, Matsumoto K, MacFarlane DR, Winther-Jensen B (2014) An investigation of the properties of conducting polymer alloys for water oxidation. Electrochim Acta 122:166–172. https://doi.org/10.1016/j.electacta.2013.10.022
Hillman AR, Ryder KS, Ismail HK, Unal A, Voorhaar A (2017) Fundamental aspects of electrochemically controlled wetting of nanoscale composite materials. Faraday Discuss 199:75–99. https://doi.org/10.1039/C7FD00060J
Hong X, Liu Y, Li Y, Wang X, Fu J, Wang X (2020) Application progress of polyaniline, polypyrrole and polythiophene in lithium-sulfur batteries. Polymers 12:331. https://doi.org/10.3390/polym12020331
Huang J, Wan M (1999) Polyaniline doped with different sulfonic acids by in situ doping polymerization. J Polym Sci Polym Chem 37:1277–1284. https://doi.org/10.1002/(SICI)1099-0518(19990501)37:9%3c1277::AID-POLA7%3e3.0.CO;2-A
Ismail HK, Alesary HF, Al-Murshedi AY, Kareem JH (2019a) Ion and solvent transfer of polyaniline films electrodeposited from deep eutectic solvents via EQCM. J Solid State Electrochem 23:3107–3121. https://doi.org/10.1007/s10008-019-04415-1
Ismail HK, Alesary HF, Mohammed MQ (2019b) Synthesis and characterisation of polyaniline and/or MoO2/graphite composites from deep eutectic solvents via chemical polymerisation. J Polym Res 26:65. https://doi.org/10.1007/s10965-019-1732-6
Jamal R, Abdiryim T, Nurulla I (2008) Comparative studies of solid-state synthesized poly (o-methoxyaniline) and poly (o-toluidine). Polym Adv Technol 19:1461–1466. https://doi.org/10.1002/pat.1139
Leclerc M, D’Aprano G, Zotti G (1993) Structure-property relationships in polyaniline derivatives. Synth Met 55:1527–1532. https://doi.org/10.1016/0379-6779(93)90279-6
Li Q, Liu J, Zou J, Chunder A, Chen Y, Zhai L (2011) Synthesis and electrochemical performance of multi-walled carbon nanotube/polyaniline/MnO2 ternary coaxial nanostructures for supercapacitors. J Power Sources 196:565–572. https://doi.org/10.1016/j.jpowsour.2010.06.073
Mattoso L, Bulhoes L (1992) Synthesis and characterization of poly (o-anisidine) films. Synth Met 52:171–181. https://doi.org/10.1016/0379-6779(92)90305-3
Mažeikien R, Malinauskas A (2002) Electrochemical stability of polyaniline. Eur Polymer J 38:1947–1952. https://doi.org/10.1016/S0014-3057(02)00103-9
Mello SV, Mattoso LHC, Santos JR Jr, Goncalves D, Faria RM, Oliveira ON Jr (1995) Electrochemical response of poly (o-ethoxyaniline) films produced by different techniques. Electrochim Acta 40:1851–1855. https://doi.org/10.1016/0013-4686(95)00130-7
Mettai B et al (2018) In situ chemical deposition of PPy/NDSA and PPy/DBSA layers on QCM electrodes: synthesis, structural, morphological and ammonia sensing performances study. J Polym Res 25:95. https://doi.org/10.1007/s10965-018-1500-z
Nie G, Zhang Y, Xu J, Zhang S (2008) Low-potential facile electrosyntheses of free-standing poly (5-methoxyindole) film with good fluorescence properties. J Electroanal Chem 622:121–127. https://doi.org/10.1016/j.jelechem.2008.05.008
Omastová M, Mičušík M (2012) Polypyrrole coating of inorganic and organic materials by chemical oxidative polymerisation. Chem Pap 66:392–414. https://doi.org/10.2478/s11696-011-0120-4
Qu K, Bai Y, Gao X, Deng M (2020) Application of poly (aniline-co-o-methoxyaniline) as energy storage material. Synth Met 262:116346. https://doi.org/10.1016/j.synthmet.2020.116346
Ren F, Zhou W, Du Y, Yang P, Wang C, Xu J (2011) High efficient electrocatalytic oxidation of formic acid at Pt dispersed on porous poly (o-methoxyaniline). Int J Hydrog Energy 36:6414–6421. https://doi.org/10.1016/j.ijhydene.2011.02.143
Sazou D, Deshpande PP (2017) Conducting polyaniline nanocomposite-based paints for corrosion protection of steel. Chem Pap 71:459–487. https://doi.org/10.1007/s11696-016-0044-0
Shinde VP, Patil PP (2013) A study on the electrochemical polymerization, characterization, and corrosion protection of o-toluidine on steel. J Solid State Electrochem 17:29–41. https://doi.org/10.1007/s10008-012-1847-8
Tian Y et al (2017) A comprehensive study of electrochromic device with variable infrared emissivity based on polyaniline conducting polymer. Sol Energy Mater Sol Cells 170:120–126. https://doi.org/10.1016/j.solmat.2017.05.053
Tseghai GB, Mengistie DA, Malengier B, Fante KA, Van Langenhove L (2020) PEDOT: PSS-Based conductive textiles and their applications. Sensors 20:1881. https://doi.org/10.3390/s20071881
Wei Y, Focke WW, Wnek GE, Ray A, MacDiarmid AG (1989) Synthesis and electrochemistry of alkyl ring-substituted polyanilines. J Phys Chem 93:495–499. https://doi.org/10.1021/j100338a095
Yalçınkaya S, Tüken T, Yazıcı B, Erbil M (2010) Electrochemical synthesis and corrosion behaviour of poly (pyrrole-co-o-anisidine-co-o-toluidine). Curr Appl Phys 10:783–789. https://doi.org/10.1016/j.cap.2009.09.015
Yin X, Ding J, Zhang S, Kong J (2006) Enantioselective sensing of chiral amino acids by potentiometric sensors based on optical active polyaniline films. Biosens Bioelectron 21:2184–2187. https://doi.org/10.1016/j.bios.2005.10.010
Zhang Z, Wei Z, Zhang L, Wan M (2005) Polyaniline nanotubes and their dendrites doped with different naphthalene sulfonic acids. Acta Mater 53:1373–1379. https://doi.org/10.1016/j.actamat.2004.11.030
Acknowledgements
HA wish to acknowledge the financial support of the Ministry of Higher Education and Scientific Research, Iraq, and would also like to thank Prof. Peter Food, for measuring conductivities of samples.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alesary, H.F., Ismail, H.K., Mohammed, M.Q. et al. A comparative study of the effect of organic dopant ions on the electrochemical and chemical synthesis of the conducting polymers polyaniline, poly(o-toluidine) and poly(o-methoxyaniline). Chem. Pap. 75, 5087–5101 (2021). https://doi.org/10.1007/s11696-020-01477-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01477-8