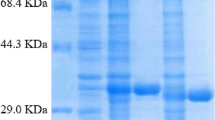
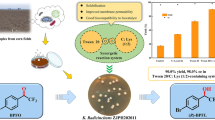
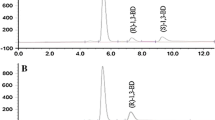
Abstract
Chiral heterocyclic secondary alcohols have received much attention due to their widespread use in pharmaceutical intermediates. In this study, Lactobacillus kefiri P2 biocatalysts isolated from traditional dairy products, were used to catalyze the asymmetric reduction of prochiral ketones to chiral secondary alcohols. Secondary chiral carbinols were obtained by asymmetric bioreduction of different prochiral substrates with results up to > 99% enantiomeric excess (ee). (R)-1-(benzofuran-2-yl)ethanol 5a, which can be used in the synthesis of pharmaceuticals such as bufuralols potent nonselective β-blockers antagonists, Amiodarone (cardiac anti-arrhythmic), and Benziodarone (coronary vasodilator), was produced in gram-scale, high yield and enantiomerically pure form using L. kefiri P2 biocatalysts. The gram-scale production was carried out, and 9.70 g of (R)-5a in enantiomerically pure form was obtained in 96% yield. Also, production of (R)-5a in terms of yield and gram scale through catalytic asymmetric reduction using the biocatalyst was the highest report so far. This is a cost-effective, clean and eco-friendly process for the preparation of chiral secondary alcohols compared to chemical processes. From an environmental and economic perspective, this biocatalytic method has great application potential, making it a green and sustainable way of synthesis.
Graphical Abstract


Similar content being viewed by others
References
Aboul-Enein MN, El-Azzouny AA, Attia MI, Maklad YA, Amin KM, Abdel-Rehim M, El-Behairy MF (2012) Design and synthesis of novel stiripentol analogues as potential anticonvulsants. Eur J Med Chem 47:360–369. https://doi.org/10.1016/j.ejmech.2011.11.004
Aldabalde V, Arcia P, Gonzalez A, Gonzalez D (2007) Enzymatic synthesis of chiral heteroaryl alcohols using plant fragments as the only biocatalyst and reducing agent. Green Chem Lett Rev 1:25–30. https://doi.org/10.1080/17518250701756983
Alizadeh BH, Foroumadi A, Emami S, Khoobi M, Panah F, Ardestani SK, Shafiee A (2010) Isochaihulactone analogues: synthesis and anti-proliferative activity of novel dibenzylbutyrolactones. Eur J Med Chem 45:5979–5984. https://doi.org/10.1016/j.ejmech.2010.09.064
Amidjojo M, Weuster-Botz D (2005) Asymmetric Synthesis of the Chiral Synthon Ethyl (S)-4-Chloro-3-Hydroxybutanoate Using Lactobacillus Kefiri. Tetrahedron:Asymmetry16: 899–901. https://doi.org/10.1016/j.tetasy.2005.01.013
Baydaş Y, Dertli E, Şahin E (2020) Green synthesis of chiral aromatic alcohols with Lactobacillus kefiri P2 as a novel biocatalyst. Synth Commun 50:1035–1045. https://doi.org/10.1080/00397911.2020.1729809
Blume F, Liu YC, Thiel D, Deska J (2016) Chemoenzymatic total synthesis of (+)- & (−)-cis-osmundalactone. J Mol Catal B Enzym 134:280–284. https://doi.org/10.1016/j.molcatb.2016.11.010
Chen X, Lu Z (2016) Iminophenyl oxazolinylphenylamine for enantioselective cobalt-catalyzed hydrosilylation of aryl ketones. Org Lett 18:4658–4661. https://doi.org/10.1021/acs.orglett.6b02260
Corey EJ, Helal CJ (1996) Asymmetric synthesis of (S)-carbinoxamine. New aspects of oxazaborolidine-catalyzed enantioselective carbonyl reduction. Tetrahedron Lett 37:5675–5678. https://doi.org/10.1016/0040-4039(96)01198-7
Feng W, Satyanarayana M, Tsai YC, Liu AA, Liu LF, LaVoie EJ (2009) Novel topoisomerase I-targeting antitumor agents synthesized from the N,N,N-trimethylammonium derivative of ARC-111,5H–2,3-dimethoxy-8,9-methylenedioxy-5-[(2-N, N, N-trimethylammonium)-ethyl]di-benzo[c, h][1,6]naphthyridin-6-one iodide. Eur J Med Chem 44:3433–3438. https://doi.org/10.1016/j.ejmech.2009.02.004
Goudarshivannanavar BC, Jayadevappa H, Mahadevan KM (2009) A convenient synthesis of 2(2-benzo[b]furo)indoles and benzofuropyrazoles. Indian J Chem 48:1419–1423
He P, Zheng H, Liu X, Lian X, Lin L, Feng X (2014) Asymmetric reduction of amino ketones with a KBH4 solution catalyzed by chiral lewis acids. Chem A Eur J 20:13482–13486. https://doi.org/10.1002/chem.201404732
Honda K, Inoue M, Ono T, Okano K, Dekishima Y, Kawabata H (2017) Improvement of operational stability of Ogataea minuta carbonyl reductase for chiral alcohol production. J Biosci Bioeng 123:673–678. https://doi.org/10.1016/j.jbiosc.2017.01.016
Kang BC, Shin SH (2017) Highly enantioselective hydrosilylation of ketones catalyzed by a chiral oxazaborolidinium ion. Org Lett 19:6316–6319. https://doi.org/10.1021/acs.orglett.7b03076
Li H, Zhu D, Hua L, Biehl ER (2009) Carbonyl reductase from Sporobolomyces salmonicolor and its mutant enzymes. Adv Synth Catal 351:583–588. https://doi.org/10.1002/adsc.200900045
Ling F, Nian S, Chen J, Luo W, Whang Z, Lv Y, Zhong W (2018) Development of ferrocene-based diamine-phosphine-sulfonamide ligands for iridium-catalyzed asymmetric hydrogenation of ketones. J Org Chem 83:10749–10761. https://doi.org/10.1021/acs.joc.8b01276
Ling F, Hou H, Chen J, Nian S, Yi X, Wang Z, Song D, Zhong W (2019) Highly enantioselective synthesis of chiral benzhydrols via manganese catalyzed asymmetric hydrogenation of unsymmetrical benzophenones using an imidazole-based chiral PNN tridentate ligand. Org Lett 21:3937–3941. https://doi.org/10.1021/acs.orglett.9b01056
Lou WY, Wang W, Smith TJ, Zong MH (2009) Biocatalytic anti-prelog stereoselective reduction of 4′-methoxyacetophenone to (R)-1-(4-methoxyphenyl)ethanol with immobilized Trigonopsis Variabilis AS2.1611 cells using an ionic liquid-containing medium. Green Chem 11:1377–1384. https://doi.org/10.1039/B823502C
Maerten E, Agbossou-Niedercorn F (2008) Preparation of pyridinyl aryl methanol derivatives by enantioselective hydrogenation of ketones using chiral Ru (diphosphine)(diamine) complexes. Attribution of their absolute configuration by 1H NMR spectroscopy using Mosher's reagent. Tetrahedron 64:8700–8708. https://doi.org/10.1016/j.tet.2008.06.104
Mangas-Sánchez J, Rodríguez-Mata M, Busto E, Gotor-Fernández V, Gotor V (2009) Chemoenzymatic synthesis of rivastigmine based on lipase-catalyzed processes. J Org Chem 74:5304–5310. https://doi.org/10.1021/jo900784g
Matsunami A, Ikeda M, Nakamura H, Yoshida M, Kuwata S, Kayaki Y (2018) Accessible Bifunctional oxy-tethered ruthenium (II) catalysts for asymmetric transfer hydrogenation. Org Lett 20:5213–5218. https://doi.org/10.1021/acs.orglett.8b02157
Mikhailine AA, Morris RH (2010) Effect of the structure of the diamine backbone of P−N−N−P ligands in iron (II) complexes on catalytic activity in the transfer hydrogenation of acetophenone. Inorg Chem 49:11039–11044. https://doi.org/10.1021/ic101548j
Muñoz Solano D, Hoyos P, Hernáiz MJ, Alcántara AR, Sánchez-Montero JM (2012) Industrial biotransformations in the synthesis of building blocks leading to enantiopure drugs. Bioresour Technol 115:196–207. https://doi.org/10.1016/j.biortech.2011.11.131
Ni Y, Li CX, Ma HM, Zhang J, Xu JH (2011) Biocatalytic properties of a recombinant aldo-keto reductase with broad substrate spectrum and excellent stereoselectivity. Appl Microbiol Biotechnol 89:1111–1118. https://doi.org/10.1007/s00253-010-2941-4
Ni Y, Xu JH (2012) Biocatalytic ketone reduction: a green and efficient access to enantiopure alcohols. Biotechnol Adv 30:1279–1288. https://doi.org/10.1016/j.biotechadv.2011.10.007
Nian S, Ling F, Chen J, Wang Z, Shen H, Yi X, Yang YF, She Y, Zhong W (2019) Highly enantioselective hydrogenation of non-ortho-substituted 2-pyridyl aryl ketones via iridium-f-diaphos catalysis. Org Lett 21:5392–5396. https://doi.org/10.1021/acs.orglett.9b01415
Ohnmacht CJ, Patel AR, Lutz RE (1971) Antimalarials. 7. Bis (trifluoromethyl)-. alpha.-(2-piperidyl)-4-quinolinemethanols. J Med Chem 14:926–928. https://doi.org/10.1021/jm00292a008
Oliveira AN, Bocca CC, Carvalho JE, Ruiz ALG, Silva TP, Rittner R, Hoehr NF (2010) New substituted 4-arylaminoquinazolines as potent inhibitors of breast tumor cell lines: in vitro and docking experiments. Eur J Med Chem 45:4339–4342. https://doi.org/10.1016/j.ejmech.2010.04.034
Pal M, Srivastava G, Moon LS, Jolly RS (2012) Bioreduction of methyl heteroaryl and aryl heteroaryl ketones in high enantiomeric excess with newly isolated fungal strains. Bioresour Technol 118:306–314. https://doi.org/10.1016/j.biortech.2012.05.031
Prasanthi G, Prasad KVSRG, Bharathi K (2013) Design, synthesis and evaluation of dialkyl 4-(benzo[d][1,3]dioxol-6-yl)-1,4-dihydro-2,6-dimethyl-1substituted pyridine-3,5-di carboxylates as potential anticonvulsants and their molecular properties prediction. Eur J Med Chem 66:516–525. https://doi.org/10.1016/j.ejmech.2013.06.006
Roy A, Bhattacharyya MS, Ravi Kumar L, Chawla HPS, Banerjee UC (2003) Microbial reduction of 1-acetonapthone: a highly efficient process for multigram synthesis of S(−)-1-(1′-napthyl) ethanol. Enzym Microb Technol 33:576–580. https://doi.org/10.1016/S0141-0229(03)00178-9
Quaglia D, Pori M, Galletti P, Emer E, Paradisi F, Giacomini D (2013) His-tagged horse liver alcohol dehydrogenase: immobilization and application in the bio-based enantioselective synthesis of (S)-arylpropanols. Process Biochem 48:810–818. https://doi.org/10.1016/j.procbio.2013.03.016
Paizs C, Toşa M, Majdik C, Moldovan P, Novak L, Kolonits P, Marcovici A, Irimie FD, Poppe L (2003) Optically active 1-(benzofuran-2-yl)ethanols and ethane-1,2-diolsby enantiotopic selective bioreductions. Tetrahedron Asymmetry 14:1495–1501. https://doi.org/10.1016/S0957-4166(03)00222-2
Ryu KC, Song LA, Lee YJ, Hong AJ, Yoon HJ, Kim A (2010) Synthesis and antifungal activity of benzofuran-5-ols. Bioorg Med Chem Lett 20:6777–6780. https://doi.org/10.1016/j.bmcl.2010.08.129
Şahin E (2020) Candida zeylanoides as whole-cell biocatalyst to perform asymmetric bioreduction of benzophenone derivatives. Synth Commun 50:612–619. https://doi.org/10.1080/00397911.2019.1710213
Şahin E (2019) Green synthesis of enantiopure (S)-1-(benzofuran-2-yl)ethanol by whole-cell biocatalyst. Chirality 31:892–897. https://doi.org/10.1002/chir.23123
Şahin E (2018) Production of (R)-1-(1, 3-benzodioxol-5-yl) ethanol in high enantiomeric purity by Lactobacillus paracasei BD 101. Chirality 30:189–194. https://doi.org/10.1002/chir.22782
Şahin E, Dertli E (2019) Biocatalyzed enantiomerically pure production of (S)-phenyl (thiophen-2-yl) methanol. J Heterocycl Chem 56:2884–2888. https://doi.org/10.1002/jhet.3681
Şahin E, Dertli E (2017) Highly enantioselective production of chiral secondary alcohols with Candida zeylanoides as a new whole cell biocatalyst. Chem Biodivers 14:e1700121. https://doi.org/10.1002/cbdv.201700121
Şahin E, Serencam H, Dertli E (2019a) Production of enantiomerically pure (S)-phenyl (pyridin-2-yl) methanol with Lactobacillus paracasei BD101. Biocatal Biotransform 37:448–454. https://doi.org/10.1080/10242422.2019.1602611
Şahin E, Serencam H, Dertli E (2019b) Whole cell application of Lactobacillus paracasei BD101 to produce enantiomerically pure (S)-cyclohexyl (phenyl) methanol. Chirality 31:211–218. https://doi.org/10.1002/chir.23048
Salvi L, Kim JG, Walsh PJ (2009) Practical catalytic asymmetric synthesis of diaryl-, aryl heteroaryl-, and diheteroarylmethanols. J Am Chem Soc 131:12483–12493. https://doi.org/10.1021/ja9046747
Salvi NA, Chattopadhyay S (2016) Laboratory scale-up synthesis of chiral carbinols using Rhizopus arrhizus. Tetrahedron Asymmetry 27:188–192. https://doi.org/10.1016/j.tetasy.2016.01.008
Schmidt S, Pedroso de Almeida T, Rother D, Hollmann F (2017) Towards environmentally acceptable synthesis of chiral α-hydroxy ketones via oxidase-lyase cascades. Green Chem 19:1226–1229. https://doi.org/10.1039/C7GC00020K
Shrivas P, Pratap U (2019) Biocatalytic one-pot three-component synthesis of 4H-chromene derivatives in non-aqueous medium. Chem Pap 73:1301–1307. https://doi.org/10.1007/s11696-018-00679-5
Sokeirik YS, Mori H, Omote M, Sato K, Tarui A (2007) Synthesis of a fluorous ligand and its application for asymmetric addition of dimethylzinc to aldehydes. Org Lett 9:1927–1929. https://doi.org/10.1021/ol070466n
Takemoto M, Achiwa K (1995) The synthesis of optically active pyridyl alcohols from the corresponding racemates by Catharanthus roseus cell cultures. Tetrahedron Asymmetry 6:2925–2928. https://doi.org/10.1016/0957-4166(95)00388-6
Takemoto M, Moriyasu Y, Achiwa K (1995) Synthesis of optically active α-phenyl pyridyl methanols with cell cultures of Nicotiana tabacum. Chem Pharm Bull 43:1458–1461. https://doi.org/10.1248/cpb.43.1458
Takemoto M, Tanaka K (2001) Synthesis of optically active α-phenylpyridylmethanols by Camellia sinensis cell culture. J Mol Catal B Enzym 15:173–176. https://doi.org/10.1016/S1381-1177(01)00021-2
Tan AWI, Fischbach M, Huebner H, Buchholz R, Hummel W, Daussmann T, Wandrey C, Liese A (2006) Synthesis of enantiopure (5R)-hydroxyhexane-2-one with immobilised whole cells of Lactobacillus Kefiri. Appl Microbiol Biotechnol 71:289–293. https://doi.org/10.1007/s00253-005-0168-6
Thomson MI, Nichol GS, Lawrence AL (2017) Total synthesis of (−)-angiopterlactone B. Org Lett 19:2199–2201. https://doi.org/10.1021/acs.orglett.7b00929
Touchard F, Bernard M, Fache F, Lemaire M (1999) Ureas and Thioureas as Rh-ligands for the enantioselective hydride transfer reduction of acetophenone. J Mol Catal A Chem 140:1–11. https://doi.org/10.1016/S1381-1169(98)00212-X
Toşa MI, Podea PV, Paizs C, Florin Dan Irimie, FD (2008) Chemoenzymatic synthesis of (R)-and (S)-1-heteroarylethanols. Tetrahedron:Asymmetry. 19:2068–2071. https://doi.org/10.1016/j.tetasy.2008.08.023
Utepova IA, Serebrennikova PO, Streltsova MS, Musikhina AA, Fedorchenko TG, Chupakhin ON, Antonchick AP (2018) Enantiomerically enriched 1, 2-P, N-bidentate ferrocenyl ligands for 1, 3-dipolar cycloaddition and transfer hydrogenation reactions. Molecules 23:1311. https://doi.org/10.3390/molecules23061311
Wang Y, Zong H, Huang H, Song L (2017) Chiral thiophosphoramide catalyzed asymmetric aryl transfer reactions for the synthesis of functional diarylmethanols. Tetrahedron Asymmetry 28:90–97. https://doi.org/10.1016/j.tetasy.2016.11.011
Wani MY, Bhat AR, Azam A, Choi I, Athar F (2012) Probing the antiamoebic and cytotoxicity potency of novel tetrazole and triazine derivatives. Eur J Med Chem 48:313–320. https://doi.org/10.1016/j.ejmech.2011.12.033
Weckbecker A, Hummel W (2006) Cloning, expression, and characterization of an (R)-specific alcohol dehydrogenase from Lactobacillus Kefiri. Biocatal Biotransform 24:380–389. https://doi.org/10.1080/10242420600893827
Wu S, Shen J, Zhou X, Chen J (2007) A novel enantioselective epoxide hydrolase for (R)-phenyl glycidyl ether to generate (R)-3-phenoxy-1,2-propanediol. Appl Microbiol Biotechnol 76:1281–1287. https://doi.org/10.1007/s00253-007-1098-2
Wu W, Liu S, Duan M, Tan X, Chen C, Xie Y, Lan Y (2016) Iridium catalysts with f-amphox ligands: asymmetric hydrogenation of simple ketones. Org Lett 18:2938–2941. https://doi.org/10.1021/acs.orglett.6b01290
Xie Y, Xu JH, Lu WY, Lin GQ (2009) Adzuki bean: a new resource of biocatalyst for asymmetric reduction of aromatic ketones with high stereoselectivity and substrate tolerance. Bioresour Tech 100:2463–2468. https://doi.org/10.1016/j.biortech.2008.11.054
Yadav GD, Devendran S (2012a) Lipase catalyzed kinetic resolution of (±)-1-(1-naphthyl) ethanol under microwave irradiation. J Mol Catal B Enzym 81:58–65. https://doi.org/10.1016/j.molcatb.2012.05.007
Yadav GD, Devendran S (2012b) Lipase catalyzed synthesis of cinnamyl acetate via transesterification in non-aqueous medium. Process Biochem 47:496–502. https://doi.org/10.1016/j.procbio.2011.12.008
Yanga W, Xua JH, Pana J, Xua Y, Wang ZL (2008) Efficient reduction of aromatic ketones with NADPH regeneration by using crude enzyme from Rhodotorula cells and mannitol as cosubstrate. Biochem Eng J 42:1–5. https://doi.org/10.1016/j.bej.2008.04.014
Yeo H, Li Y, Fu L, Zhu JL, Gullen EA, Dutschman GE, Lee Y, Chung R, Huang ES, Austin DJ, Cheng YC (2005) Synthesis and antiviral activity of helioxanthin analogues. J Med Chem 48:534–546. https://doi.org/10.1021/jm034265a
Yılmaz D, Şahin E, Dertli E (2017) Highly enantioselective production of chiral secondary alcohols using Lactobacillus paracasei BD 101 as a new whole cell biocatalyst and evaluation of their antimicrobial effects. Chem Biodivers 14:e1700269. https://doi.org/10.1002/cbdv.201700269
Yu S, Li H, Lu Y, Zheng G (2018) A catalyst from burkholderia cenocepacia for efficient anti-prelog’s bioreduction of 3,5-bis(trifluoromethyl) acetophenone. Appl Biochem Biotechnol 184:1319–1331. https://doi.org/10.1007/s12010-017-2628-8
Zhou M, O’Doherty GA (2008) De novo synthesis of the trisaccharide subunit of landomycins A and E. Org Lett 10:2283–2286. https://doi.org/10.1021/ol800697k
Zhou X, Wu X, Yang B, Xiao J (2012) Varying the ratio of formic acid to triethylamine impacts on asymmetric transfer hydrogenation of ketones. J Mol Catal A Chem 357:133–140. https://doi.org/10.1016/j.molcata.2012.02.002
Acknowledgements
The authors thank Dr. Enes DERTLİ (Yildiz Technical University) for providing the bacteria strain used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baydaş, Y., Kalay, E. & Şahin, E. Asymmetric reduction of aromatic heterocyclic ketones with bio-based catalyst Lactobacillus kefiri P2. Chem. Pap. 75, 1147–1155 (2021). https://doi.org/10.1007/s11696-020-01364-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01364-2