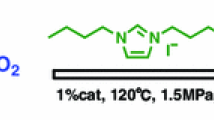
Abstract
The ionic liquid chlorination (1-amino-2-propyl alcohol)-3-buthylimidazole containing amino and hydroxy from the difunctional group is newly synthesized, the characterization of which is analyzed with NMR. It can catalyze CO2 and propylene oxide (PO) under certain conditions for preparation of propylene carbonate, with the conversion of 80.1% and the selectivity of 92.5%, which has superior performance to that of monoamino ionic liquid. The density functional theory and the quantum theory of atoms in molecules are adopted to theoretically analyze the stable structure and interactive relationship. According to the results of quantum chemical calculation, it is concluded that the functional groups of amino and hydroxy on IL are coupled with PO to generate hydrogen bond, based on which the mechanism of cycloaddition reaction can be inferred.





Similar content being viewed by others
References
Aldaco R, Butnar I, Margallo M, Laso J, Rumayor M, Dominguez-Ramos A, Irabien A, Dodds PE (2019) Bringing value to the chemical industry from capture, storage and use of CO2: a dynamic LCA of formic acid production. Sci Total Environ 663:738–753. https://doi.org/10.1016/j.scitotenv.2019.01.395
Algarasiller G, Severin N, Chong SY, Bjorkman T, Palgrave RG, Laybourn A, Antonietti M, Khimyak YZ, Krasheninnikov AV, Rabe JP, Kaiser U, Cooper AI, Thomas A, Bojdys MJ (2014) Triazine-based graphitic carbon nitride: a two-dimensional semiconductor. Angewandte Chem 126:7580–7585. https://doi.org/10.1002/anie.201402191
Babucci M, Fang CY, Hoffman AS, Bare SR (2017) Tuning the selectivity of single-site supported metal catalysts with ionic liquids. ACS Catal 7:6969–6972. https://doi.org/10.1021/acscatal.7b02429
Bo L, Jianmin S (2019) Fixation of CO2 into cyclic carbonates catalyzed by Zn-based heterogeneous catalysts. Harbin Institute of Technology, Harbin
Chen C, Ma Y, Zheng D, Wang L, Li JF, Zhang JL, He HY, Zhang SJ (2017) Insight into the role of weak interaction played in the fixation of CO2, catalyzed by the amino-functionalized imidazolium-based ionic liquids. J Utilization 18:156–163. https://doi.org/10.1016/j.jcou.2017.01.026
Clements JH (1994) Reactive applications of cyclic alkylene carbonates. Ind Eng Chem Res 42:663–674. https://doi.org/10.1021/ie020678i
Dai WL, Luo SL, Yin SF, Au CT (2009) The direct transformation of carbon dioxide to organic carbonates over heterogeneous catalysts. Appl Catal Gen 366:2–12. https://doi.org/10.1016/j.apcata.2009.06.045
Decortes A, Castilla AM, Kleij AW (2010) Salen-complex-mediated formation of cyclic carbonates by cycloaddition of CO2 to epoxides. Angewandte Chem 49:9822–9837. https://doi.org/10.1002/anie.201002087
Desai SH, Atsumi S (2013) Photosynthetic approaches to chemical biotechnology. Curr Opin Biotechnol 24:1031–1036. https://doi.org/10.1016/j.copbio.2013.03.015
Dzyuba SV, Bartsch RA (2001) Influence of structural variations in 1-alkyl(aralkyl)-3-methylimidazolium hexafluorophosphates and bis (trifluoromethylsulfonyl) imides on physical properties of the ionic liquids. ChemPhysChem 3:161–166. https://doi.org/10.1002/1439-7641(20020215)3:2%3c161:aid-cphc161%3e3.0.co;2-3
Fazekas E, Nichol GS, Shaver MP, Garden JA (2018) Stable Fe(iii) phenoxyimines as selective and robust CO2/epoxide coupling catalysts. Dalton Trans 47:13106–13112. https://doi.org/10.1039/C8DT02919A
Gao HX, Chen GY, Fu KY, Liu HL, Tong BD, Liang WZ (2012) Highly efficient synthesis of cyclic carbonates from CO2 and propylene epoxide catalyzed by Bu4NBr/Mg(OH)Cl. Chem Ind Eng Prog 31:240–242. https://doi.org/10.16085/j.issn.1000-6613.2012.s1.032
Guo Z, Cai X, Xie J, Wang X, Zhou Y, Wang J (2016) Hydroxyl-exchanged nanoporous ionic copolymer toward low-temperature cycloaddition of atmospheric carbon dioxide into carbonates. ACS Appl Mater Interfaces 20:12812–12821. https://doi.org/10.1021/acsami.6b02461
James B, Frisch A (1996) Exploring chemistry with electronic structure methods. Gaussian Incorporated, Wallingford
Kai W, Zhanghuai S, Hongying L (2019) The application of deep eutectic solvent in the synthesis of propylene carbonate. Yantai University, Yantai
Kim HU, Babu R, Roshan R, Park DW (2017) Catalytic performance of metal azolate frameworks in the solventless synthesis of cyclic carbonates from CO2 and epoxides. Appl Catal A General 538:59–65. https://doi.org/10.1016/j.apcata.2017.03.028
Lian X, Xu L, Chen M, Wu C, Li W, Huang B, Cui Y (2019) Carbon dioxide captured by metal organic frameworks and its subsequent resource utilization strategy: a review and prospect. J Nanosci Nanotechnol 19:3059–3078. https://doi.org/10.1166/jnn.2019.16647
Lili D (2018) Preparation of immobilized ionic liquids catalysts and their application for conversion of CO2. Shenyang University of Technology, Liaoning
Lipkowski P, Grabowski SJ, Robinson TL (2004) Properties of the C−H···H dihydrogen bond: an ab initio and topological analysis. J Phys Chem A 108:10865–10872. https://doi.org/10.1021/jp048562i
Mao H, Zhang H, Liang J, Liu D, Wu S, Zhang Y, Zhang Y, Wu Q, Zhang G, Song X-M (2015) Preparation of poly(ionic liquids)-functionalized polypyrrole nanotubes and their electrocatalytic application to simultaneously determine dopamine and ascorbic acid. J Mater Chem B 3:5310–5317. https://doi.org/10.1039/C5TB00259A
Matta CF, Huang L, Lou M (2011) Characterization of a trihydrogen bond on the basis of the topology of the electron density. J Phys Chem 115:12451–12458. https://doi.org/10.1021/jp203973d
Miao Q, Liang J, Yang X et al (2015a) Progress in the basic catalytic application of functional basic ionic liquids. Shiyou Xuebao, Shiyou Jiagong/Acta Petrolei Sinica (Petrol Process Sect) 31(1):188–193. https://doi.org/10.3969/j.issn.1001-8719.2015.01.029
Miao Q, Liang J, Yang X, Xu W, Shu Y, Zhu J (2015b) Progress in the basic catalytic application of functional basic ionic liquids. Acta Petrolei Sinica. https://doi.org/10.3969/j.issn.1001-8719.2015.0l.029
Monkman S, Macdonald M (2016) Carbon dioxide upcycling into industrially produced concrete blocks. Constr Build Mater 124:127–132. https://doi.org/10.1016/j.conbuildmat.2016.07.046
Pearson JK, Boyd RJ (2007) Density functional theory study of the reaction mechanism and energetics of the reduction of hydrogen peroxide by ebselen, ebselen diselenide, and ebselen selenol. J Phys Chem A 111(16):3152–3160. https://doi.org/10.1021/jp071499n
Peng J, Wang S, Yang HJ, Ban BR, Wei ZD, Wang LH, Lei B (2018) Highly efficient fixation of carbon dioxide to cyclic carbonates with new multi-hydroxyl bis-(quaternary ammonium) ionic liquids as metal-free catalysts under mild conditions. Fuel 224:481–488. https://doi.org/10.1016/j.fuel.2018.03.119
Qian ZH (2016) The systhesis of new containing amino dial-core basic ionic liquid and used to absorb CO2. Hebei University of Technology, Tianjin
Qiao Y, Ma W, Theyssen N, Chen C, Hou ZS (2017) Temperature-responsive ionic liquids: fundamental behaviors and catalytic applications. Chem Rev 117:6881–6928. https://doi.org/10.1021/acs.chemrev.6b00652
Ravaei I, Haghighat M, Azami SM (2019) A DFT, AIM and NBO study of isoniazid drug delivery by MgO nanocage. Appl Surf Sci 469:103–112. https://doi.org/10.1016/j.apsusc.2018.11.005
Shaobo X, Tingting L, Qiri Z, Yingqiu G, Lijuan S (2019) Mim-NH2/TBAB ionic liquid-based binary homogeneous catalyst system for efficient catalytic conversion of CO2 under mild conditions. Nat Gas Chem Ind 44(1):73–77. https://doi.org/10.3969/j.issn.1001-9219.2019.01.014
Wang L, Li P, Jin X, Zhang J, He H, Zhang S (2015) Mechanism of fixation of CO2 in the presence of hydroxyl-functionalized quaternary ammonium salts. J Utilization 10:113–119. https://doi.org/10.1016/j.jcou.2015.02.006
Wang T, Song X, Luo Q, Yang X, Chong S, Zhang J, Ji M (2018) Acid-base bifunctional catalyst: Carboxyl ionic liquid immobilized on MIL-101-NH2 for rapid synthesis of propylene carbonate from CO2 and propylene oxide under facile solvent-free conditions. Microporous Mesoporous Mater 267:84–92. https://doi.org/10.1016/j.micromeso.2018.03.011
Werner T, Tenhumberg N (2014) Synthesis of cyclic carbonates from epoxides and CO2 catalyzed by potassium iodide and amino alcohols. J Utilization 7:39–45. https://doi.org/10.1016/j.jcou.2014.04.002
Wu YL, Jiao Z, Wang GN et al (2007) Synthesis, characterization and absorption efficiency of an ionic liquid for the absorption of CO2. Fine Chem Icals 24(4):324–327. https://doi.org/10.1002/pat.1973
Xia SM, Chen KH, Fu HC, He LN (2018) Ionic liquids catalysis for carbon dioxide conversion with nucleophiles. Front Chem 6:462–468. https://doi.org/10.3389/fchem.2018.00462
Xu J, Xu M, Wu J, Wu H, Zhang WH, Li YX (2015) Graphene oxide immobilized with ionic liquids: facile preparation and efficient catalysis for solvent-free cycloaddition of CO2 to propylene carbonate. RSC Adv 5:72361–72368. https://doi.org/10.1039/c5ra13533h
Yang H, Wang X, Ma Y, Wang L, Zhang J (2016) Quaternary ammonium-based ionic liquids bearing different numbers of hydroxyl groups as highly efficient catalysts for the fixation of CO2: a theoretical study by QM and MD. Catal Sci Technol 6:7773–7782. https://doi.org/10.1039/c6cy01045h
Yue S, Wang P, Hao X, Zang S (2017) Dual amino-functionalized ionic liquids as efficient catalysts for carbonate synthesis from carbon dioxide and epoxide under solvent and cocatalyst-free conditions. J Utilization 21:238–246. https://doi.org/10.1016/j.jcou.2017.07.017
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, W., He, J., Zhang, B. et al. Preparation of propylene carbonate catalyzed by ionic liquid. Chem. Pap. 74, 2583–2590 (2020). https://doi.org/10.1007/s11696-020-01053-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-020-01053-0