Abstract
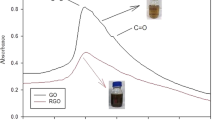
Ginkgo biloba leaves (GBL) contain many biologically active compounds such as flavonoids and terpene trilactones. In this study, graphene oxide (GO)-assisted ethanol reflux extraction (GERE) was adopted to extract the Ginkgo flavonoids (GF) from GBL, and the kinetics and mechanism of GF extraction were studied. SEM, XRD and FT-IR results all showed that GO could disrupt the cellulose structure and reduce the crystallinity of GBL. Through the obtained experimental data, it can be seen that So–Macdonald model was suitable to describe the extraction kinetics with high values of the coefficient of determination (R2 ≥ 0.994) and low values of the residual sum of squares (RSS ≤ 0.00022). The optimum leaching conditions of GERE were 70% (v/v) of ethanol concentration, 16 mL/g of liquid to solid ratio, 1.5 mg/g of GO dosage and 140 rpm of stirring speed at 70°C for 4 h by the single factor experiments. Under these conditions, the equilibrium yield of GF by GERE reached 82.63%, about 5.60% higher than the conventional ethanol reflux extraction (ERE). The value of the apparent activation energy obtained was 3.69 kJ/mol for GERE, 6.35% lower than that for ERE at a temperature within the range of 40–70 °C. HPLC results indicated that GO could not decompose flavonoids. The higher yield of GF can be attributed to the catalytic hydrolysis to the cell wall by GO, which can decrease the apparent activation energy and accelerate the leaching of GF at the fast diffusion stage.
Graphic abstract
GERE method is an efficient and green extraction technology and So–Macdonald model satisfactorily described the extraction kinetics of Ginkgo flavonoids.








Similar content being viewed by others
Abbreviations
- A :
-
Frequency factor (m2/s)
- A f :
-
Absorbance
- C e :
-
Equilibrium extract concentration (mg/mL)
- C d1 e :
-
Hypothetical equilibrium concentration at the fast diffusion stage (mg/mL)
- C d2 e :
-
Hypothetical equilibrium concentration at the slow diffusion stage (mg/mL)
- C w e :
-
Hypothetical equilibrium concentration at the washing stage (mg/mL)
- C t :
-
Extract concentration at time t (mg/mL)
- D :
-
Diffusion coefficient (m2/s)
- D′:
-
Apparent diffusion coefficient (m2/s)
- E a :
-
Activation energy (kJ/mol)
- G :
-
GO dosage (mg/g)
- k 2 :
-
Second-order rate constant (1/s)
- k d :
-
Extraction rate constant (1/s)
- k d1 :
-
Extraction rate constant for the fast diffusion stage (1/s)
- k d2 :
-
Extraction rate constant for the slow diffusion stage (1/s)
- k obs :
-
First-order rate constant (1/s)
- k w :
-
Extraction rate constant for the washing stage (1/s)
- L :
-
Length of Ginkgo biloba leaves (m)
- m :
-
Weight of dry Ginkgo biloba (g)
- n :
-
Stirring speed (rpm)
- n d :
-
Number of experimental data
- N :
-
Number of constants in the model
- p, q :
-
Model parameter factors
- R :
-
Universal gas constant 8.314 × 10−3 (kJ/mol/K)
- S :
-
Liquid-to-solid ratio (mL/g)
- t :
-
Extraction time (s)
- T :
-
Temperature (°C, K)
- T c :
-
Appropriate temperature for graphene oxide-assisted ethanol extraction (K)
- V :
-
Volume of the extraction medium (mL)
- W :
-
Ginkgo flavonoids content of dry Ginkgo biloba leaves (mg/g)
- φ :
-
Ethanol concentration (%)
- 2δ :
-
Thickness of GBL (m)
- η e :
-
Equilibrium yields of GF (%)
- η t :
-
Yields of Ginkgo flavonoids at time t (%)
- η exp,i :
-
Experimentally measured values of the extraction yield (%)
- η pre,i :
-
Predicted values of the extraction yield (%)
- \(\bar{\eta }\) :
-
Mean of measurement value of the extraction yield (%)
References
Ahlemeyer B, Krieglstein J (2003) Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci 60:1779–1792. https://doi.org/10.1007/s00018-003-3080-1
Amarante RCA, Oliveira PM, Schwantes FK, Morón Villarreyes JA (2014) Oil extraction from castor cake using ethanol: kinetics and thermodynamics. Ind Eng Chem Res 53:6824–6829. https://doi.org/10.1021/ie500508n
Amendola D, De Faveri DM, Spigno G (2010) Grape marc phenolics: extraction kinetics, quality and stability of extracts. J Food Eng 97:384–392. https://doi.org/10.1016/j.jfoodeng.2009.10.033
Campos LMAS, Michielin EMZ, Danielski L, Ferreira SRS (2005) Experimental data and modeling the supercritical fluid extraction of marigold (Calendula officinalis) oleoresin. J Supercrit Fluids 34:163–170. https://doi.org/10.1016/j.supflu.2004.11.010
Cao Y, Tan HM (2004) Structural characterization of cellulose with enzymatic treatment. J Mol Struct 705:189–193. https://doi.org/10.1016/j.molstruc.2004.07.010
Chen S, Xing XH, Huang JJ, Xu MS (2011) Enzyme-assisted extraction of flavonoids from Ginkgo biloba leaves: improvement effect of flavonol transglycosylation catalyzed by Penicillium decumbens cellulase. Enzyme Microb Technol 48:100–105. https://doi.org/10.1016/j.enzmictec.2010.09.017
Elliott M, Kandaswami C, Theoharides CT (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev 52:673–751. https://doi.org/10.1006/phrs.2000.0734
Franco D, Sineiro J, Pinelo M, Núñez MJ (2007) Ethanolic extraction of Rosa rubiginosa soluble substances: oil solubility equilibria and kinetic studies. J Food Eng 79:150–157. https://doi.org/10.1016/j.jfoodeng.2006.01.047
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21:885–896. https://doi.org/10.1007/s10570-013-0030-4
Ghasemzadeh A, Jaafar HZE (2014) Optimization of reflux conditions for total flavonoid and total phenolic extraction and enhanced antioxidant capacity in Pandan (Pandanus amaryllifolius Roxb.) using response surface methodology. Sci World J 2014:1–10. https://doi.org/10.1155/2014/523120
Gironi F, Piemonte V (2011) Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem Eng Res Des 89:857–862. https://doi.org/10.1016/j.cherd.2010.11.003
Gong QY (2019) Extraction of flavonoids from Ginkgo biloba leaves by graphene oxide-assisted ethanol reflux method and its mechanism. Dissertation, Hefei University of Technology, China
Gujar JG, Chattopadhyay S, Wagh SJ, Gaikar VG (2010) Experimental and modeling studies on extraction of catechin hydrate and epicatechin from Indian green tea leaves. Can J Chem Eng 88:232–240. https://doi.org/10.1002/cjce.20271
Ho YS, Harouna Oumarou HA, Fauduet H, Porte C (2005) Kinetics and model building of leaching of water-soluble compounds of Tilia sapwood. Sep Purif Technol 45:169–173. https://doi.org/10.1016/j.seppur.2005.03.007
Ibrahim MM, El Zawawy WK, Jüttke Y, Koschella A, Heinze T (2013) Cellulose and microcrystalline cellulose from rice straw and banana plant waste: preparation and characterization. Cellulose 20:2403–2416. https://doi.org/10.1007/s10570-013-9992-5
Imamoglu E, Sukan FV (2013) Scale-up and kinetic modeling for bioethanol production. Bioresour Technol 144:311–320. https://doi.org/10.1016/j.biortech.2013.06.118
Jaganyi D, Madlala SP (2000) Kinetics of coffee infusion: a comparative study on the extraction kinetics of mineral ions and caffeine from several types of medium roasted coffees. J Sci Food Agric 80:85–90. https://doi.org/10.1002/(SICI)1097-0010(20000101)80:1%3C85:AID-JSFA489%3E3.0.CO;2-%23
Jaganyi D, Price RD (1999) Kinetics of tea infusion: the effect of the manufacturing process on the rate of extraction of caffeine. Food Chem 64:27–31. https://doi.org/10.1016/S0308-8146(98)00101-0
Krishnan RY, Rajan KS (2016) Microwave assisted extraction of flavonoids from Terminalia bellerica: study of kinetics and thermodynamics. Sep Purif Technol 157:169–178. https://doi.org/10.1016/j.seppur.2015.11.035
Langkilde FW, Svantesson A (1995) Identification of celluloses with Fourier-Transform (FT) mid-infrared, FT-Raman and near-infrared spectrometry. J Pharm Biomed Anal 13:409–414. https://doi.org/10.1016/0731-7085(95)01298-Y
Li XZ, Zhang HY, Xu YL (2014) Study on extraction of total flavonoids from Ginkgo biloba leaves by reflux method. Chem Bioeng 31:31–34. https://doi.org/10.3969/j.issn.1672-5425.2014.03.009(in Chinese)
Linares AR, Hase SL, Vergara ML, Resnik SL (2010) Modeling yerba mate aqueous extraction kinetics: influence of temperature. J Food Eng 97:471–477. https://doi.org/10.1016/j.jfoodeng.2009.11.003
Mansour SM, Bahgat AK, El-Khatib AS, Khayyal MT (2011) Ginkgo biloba extract (EGb 761) normalizes hypertension in 2 K, 1C hypertensive rats: role of antioxidant mechanisms, ACE inhibiting activity and improvement of endothelial dysfunction. Phytomedicine 18:641–647. https://doi.org/10.1016/j.phymed.2011.01.014
Marcano DC et al (2010) Improved synthesis of graphene oxide. Acs Nano 4:4806. https://doi.org/10.1021/nn1006368
Meziane S, Kadi H (2008) Kinetics and thermodynamics of oil extraction from olive cake. J Am Oil Chem Soc 85:391–396. https://doi.org/10.1007/s11746-008-1205-2
Miao SF, Yu JP, Du Z, Guan YX, Yao SJ, Zhu ZQ (2010) Supercritical fluid extraction and micronization of Ginkgo flavonoids from Ginkgo biloba leaves. Ind Eng Chem Res 49:5461–5466. https://doi.org/10.1021/ie902001x
Naik SR, Panda VS (2007) Antioxidant and hepatoprotective effects of Ginkgo biloba phytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int 27:393–399. https://doi.org/10.1111/j.1478-3231.2007.01463.x
Nelson ML, O’Connor RT (1964) Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J Appl Polym Sci 8:1325–1341. https://doi.org/10.1002/app.1964.070080323
Niu JH et al (2018) Simultaneous determination of melatonin, l-tryptophan, and two l-tryptophan-derived esters in food by HPLC with graphene oxide/SiO2 nanocomposite as the adsorbent. Food Anal Methods 11:2438–2446. https://doi.org/10.1007/s12161-018-1213-2
Pääkkö M et al (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromol 8:1934–1941. https://doi.org/10.1021/bm061215p
Perez EE, Carelli AA, Crapiste GH (2011) Temperature-dependent diffusion coefficient of oil from different sunflower seeds during extraction with hexane. J Food Eng 105:180–185. https://doi.org/10.1016/j.jfoodeng.2011.02.025
Price WE, Spiro M (1985) Kinetics and equilibria of tea infusion: rates of extraction of theaflavin, caffeine and theobromine from several whole teas and sieved fractions. J Sci Food Agric 36:1309–1314. https://doi.org/10.1002/jsfa.2740361216
Rao CNR, Sood AK, Subrahmanyam KS, Govindaraj A (2010) Graphene: the new two-dimensional nanomaterial. Angew Chem Int Ed 48:7752–7777. https://doi.org/10.1002/anie.200901678
Simha P, Mathew M, Ganesapillai M (2016) Empirical modeling of drying kinetics and microwave assisted extraction of bioactive compounds from Adathoda vasica and Cymbopogon citratus. Alex Eng J 55:141–150. https://doi.org/10.1016/j.aej.2015.12.020
So GC, Macdonald DG (1986) Kinetics of oil extraction from canola (rapeseed). Can J Chem Eng 64:80–86. https://doi.org/10.1002/cjce.5450640112
Spiro M (1997) Factors affecting the rate of infusion of black tea. Chemical and biological properties of tea infusions. Unwelt and Medizin Publishers, Frankfurt, pp 38–62
Spiro M, Siddique S (1981) Kinetics and equilibria of tea infusion: kinetics of extraction of theaflavins, thearubigins and caffeine from Koonsong broken pekoe. J Sci Food Agric 32:1135–1139. https://doi.org/10.1002/jsfa.2740321115
Stapley AGF (2002) Modelling the kinetics of tea and coffee infusion. J Sci Food Agric 82:1661–1671. https://doi.org/10.1002/jsfa.1250
Thobani M, Diosady LL (1997) Two-phase solvent extraction of canola. J Am Oil Chem Soc 74:207–214. https://doi.org/10.1007/s11746-997-0125-x
Toda TA, Sawada MM, Rodrigues CEC (2016) Kinetics of soybean oil extraction using ethanol as solvent: experimental data and modeling. Food Bioprod Process 98:1–10. https://doi.org/10.1016/j.fbp.2015.12.003
Wang X, Wei FY (2017) Kinetic study of application of graphene oxide as a catalyst to accelerate extraction of total flavonoids from Radix Scutellaria. RSC Adv 7:46894–46899. https://doi.org/10.1039/c7ra06183h
Wang LL, Han GT, Zhang YM (2007) Comparative study of composition, structure and properties of Apocynum venetum fibers under different pretreatments. Carbohydr Polym 69:391–397. https://doi.org/10.1016/j.carbpol.2006.12.028
Wang BW, Li JJ, Gong QY, Wei FY, Xu ZQ, Xu YB (2017) Graphene oxide-assisted ethanol reflux extraction of flavonoids in bamboo leaves. Chin J Process Eng 17:709–715. https://doi.org/10.12034/j.issn.1009-606X.216348(in Chinese)
Wei FY, Chen W, Fang J, Bao HH (2013) Mass transfer kinetics of flavonoids extraction from bamboo leaves with cellulase-assisted aqueous extractant. J Chem Eng Chin Univ 27:779–784. https://doi.org/10.3969/j.issn.1003-9015.2013.05.009(in Chinese)
Wei FY, Wang X, Zhang XY (2014) Application of graphene oxide as a catalyst to accelerate extraction of total flavonoids and the hydrolysis of baicalin from Radix Scutellaria. Sep Purif Technol 133:421–428. https://doi.org/10.1016/j.seppur.2014.06.062
Xi J, Yan LG (2017) Optimization of pressure-enhanced solid-liquid extraction of flavonoids from Flos Sophorae and evaluation of their antioxidant activity. Sep Purif Technol 175:170–176. https://doi.org/10.1016/j.seppur.2016.10.013
Xie JL, Hse CY, De Hoop CF, Hu TX, Qi JQ, Shupe TF (2016) Isolation and characterization of cellulose nanofibers from bamboo using microwave liquefaction combined with chemical treatment and ultrasonication. Carbohydr Polym 151:725–734. https://doi.org/10.1016/j.carbpol.2016.06.011
Zhang YQ, Chen AY, Li M, Chen CY, Yao QZ (2008) Ginkgo biloba extract kaempferol inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. J Surg Res 148:17–23. https://doi.org/10.1016/j.jss.2008.02.036
Zhang WW, Xu F, Cheng H, Li LL, Cao FL, Cheng SY (2013) Effect of chlorocholine chloride on chlorophyll, photosynthesis, soluble sugar and flavonoids of Ginkgo biloba. Not Bot Horti Agrobo 41:97–103. https://doi.org/10.15835/nbha4118294
Zhang XN, Niu JH, Zhang XT, Xiao R, Lu MH, Cai ZW (2017) Graphene oxide-SiO2 nanocomposite as the adsorbent for extraction and preconcentration of plant hormones for HPLC analysis. J Chromatogr B 1046:58–64. https://doi.org/10.1016/j.jchromb.2017.01.004
Zhao CJ et al (2011) Micronization of Ginkgo biloba extract using supercritical antisolvent process. Powder Technol 209:73–80. https://doi.org/10.1016/j.powtec.2011.02.011
Zhao LJ et al (2018) Determination of total flavonoids contents and antioxidant activity of Ginkgo biloba leaf by near-infrared reflectance method. Int J Anal Chem 2018:1–7. https://doi.org/10.1155/2018/8195784
Zheng AB (2007) The studies of the fingerprints in Ginkgo leaf and its application in quality analysis of Ginkgo leaf preparation. South Cent. Univ. Natl., China
Zu YG et al (2016) Purification of Ginkgo biloba extract by antisolvent recrystallization. Chem Eng Technol 39:1301–1308. https://doi.org/10.1002/ceat.201500691
Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 51372062).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gong, Q., Guo, Z., Sun, Z. et al. Graphene oxide-assisted ethanol reflux extraction of total flavonoids from Ginkgo biloba leaves: study of kinetics and mechanism. Chem. Pap. 74, 971–984 (2020). https://doi.org/10.1007/s11696-019-00934-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00934-3