Abstract
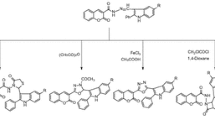
A series of novel 2,3-di-substituted-2,3-dihydro-quinazolin-4(1H)-one derived Schiff’s bases (1–7) have been designed, synthesized and characterized on the basis of their physical and spectral data. The presented microwave-assisted, phosphomolybdic acid (PMoA) catalysed, protocol provides an efficient and convenient route for the synthesis of structurally diverse and potentially biologically active compounds. The molecular structures of these Schiff’s bases related to quinazolinones were confirmed by various spectroscopic methods (NMR, FTIR, UV–Vis) and their antioxidant activities were evaluated by UV–Vis and EPR spectroscopy employing 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assay. Derivatives 1–7 were examined for their cytotoxicity in vitro against human hepatocellular carcinoma cells (HepG2) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphentltetrazolium bromide (MTT) assay. The structure–activity relationships study revealed that the position and nature of functional groups attached to the quinazolinone moiety alter their physico-chemical and biological properties. Derivatives 5, 6 and 7 bearing multiple electron-donating groups were found to be the most active members of this series.





Similar content being viewed by others
References
Berridge MV, Herst PM, Tan AS (2005) Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Ann Rev 11:127–152. https://doi.org/10.1016/S1387-2656(05)11004-7
Besson T, Chosson E (2007) Microwave-assisted synthesis of bioactive quinazolines and quinazolinones. Comb Chem High Throughput Screen 10:903–917. https://doi.org/10.2174/138620707783220356
Blevins AA, Blanchard GJ (2004) Effect of positional substitution on the optical response of symmetrically disubstituted azobenzene derivatives. J Phys Chem B 108:4962–4968. https://doi.org/10.1021/jp037436w
Brees KD, Lamѐthe JF, DeArmitt C (2000) Improving synthetic hindered phenol antioxidants:learning from vitamin E. Polym Degrad Stab 70:89–96. https://doi.org/10.1016/S0141-3910(00)00094-X
Chawla A, Batra Ch (2013) Recent advances of quinazolinone derivatives as marker for various biological activities. Int Res J Pharm 4:49–58. https://doi.org/10.7897/2230-8407.04309
Cozzi PG (2004) Metal-Salen schiff base complexes in catalysis: practical aspects. Chem Soc Rev 33:410–421. https://doi.org/10.1039/B307853C
Crompton EM, Lewis FD (2004) Positional effects of the hydroxy substituent on the photochemical and photophysical behavior of 3- and 4-hydroxystilbene. Photochem Photobiol Sci 3:660–668. https://doi.org/10.1039/b403661a
Da Silva CM, Da Silva DL, Modolo LV, Alves RB, De Resende MA, Martins CVB, De Fatima A (2011) Schiff bases: a short review of their antimicrobial activities. J Adv Res 2:1–8. https://doi.org/10.1016/j.jare.2010.05.004
Díaz MS, Freile ML, Gutiérrez MI (2009) Solvent effect on the UV/Vis absorption and fluorescence spectroscopic properties of berberine. Photochem Photobiol Sci 8:970–974. https://doi.org/10.1039/b822363g
Ernst OP, Lodowski DT, Elstner M, Hegemann P, Brown LS, Kandori H (2014) Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem Rev 114:126–163. https://doi.org/10.1021/r4003769c
Firouzabadi H, Jafari AA (2005) Heteropoly acids, their salts and polyoxometalates as heterogenous, efficient and eco-friendly catalysts in organic reactions: Some recent advances. J Iran Chem Soc 2:85–114. https://doi.org/10.1007/BF03247201
Fülöp F, Simeonov M, Pihlaja K (1992) Formation of 1,2-dihydroquinazolin-4(3H)-ones. Reinvestigation of a recently reported 1,3,-4-benzotriazepine synthesis. Tetrahedron 48:531–538. https://doi.org/10.1016/S0040-4020(01)89014-1
Gawad ANM, Georgey HH, Youssef RM, El-sayed NA (2010) Synthesis and antitumor activity of some 2,3-disubstituted quinazolin-4(3H)-ones and 4,6-disubstituted-1,2,3,4 tetrahydroquinazolin-2H-ones. Eur J Med Chem 45:6058–6067. https://doi.org/10.1016/j.ejmech.2010.10.008
Guo Z, Xing R, Liu S, Zhong Z, Ji X, Wang L, Li P (2007) Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydr Res 342:1329–1332. https://doi.org/10.1016/j.carres.2007.04.006
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Clarendon Press, Oxford
Hricovíni M, Hricovíni M (2017) Photochemically-induced anti-syn isomerization of quinazolinone-derived Schiff’s bases: EPR, NMR and DFT analysis. Tetrahedron 73:252–261. https://doi.org/10.1016/j.tet.2016.12.011
Hricovíniová Z (2006) The effect of microwave irradiation on Mo(VI) catalysed transformations of reducing saccharides. Carbohydr Res 341:2131–2134. https://doi.org/10.1016/j.carres.2006.05.007
Hricovíniová Z (2010) A new approach to Amadori ketoses via Mo(VI)-catalyzed stereospecific isomerization of 2-C-branched sugars bearing azido function in a microwave field. Tetrahedron Asymm 21:2238–2243. https://doi.org/10.1016/j.tetasy.2010.07.027
Hricovíniová Z (2016) Surfactants of biological origin: the role of MO(VI) and microwaves in the synthesis of xylan-based non-ionic surfactants. Carbohydr Polym 144:297–304. https://doi.org/10.1016/j.carbpol.2016.02.070
Kajiyama T, Ohkatsu Y (2001) Effect of para-substitutes of phenolic antioxidants. Polym Degrad Stab 71:445–452. https://doi.org/10.1016/S0141-3910(00)00196-8
Kaur H, Limb SM, Ramasamy K, Vasudevan M, Shah SAA, Narasimhan B (2017) Diazenyl schiff bases: synthesis, spectral analysis, antimicrobial studies and cytotoxic activity on human colorectal carcinoma cell line (HCT-116). Arab J. Chem. https://doi.org/10.1016/j.arabjc.2017.05.004
Khan MTH, Khan R, Wuxiuer Y, Arfan M, Ahmed M, Sylte I (2010) Identification of novel quinazolin-4 (3H)-ones as inhibitors of thermolysin, the prototype of the M4 family of proteinases. Bioorg Med Chem 18:4317–4327. https://doi.org/10.1016/j.bmc.2010.04.083
Kozhevnikov IV (1998) Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem Rev 98:171–198. https://doi.org/10.1021/cr960400y
Kumar A, Sharma P, Kumari P, Lal Kalal B (2011) Exploration of antimicrobial and antioxidant potential of newly synthesized 2,3-disubstituted quinazoline-4(3H)-ones. Bioorg Med Chem Lett 21:4353–4357. https://doi.org/10.1016/j.bmcl.2011.05.031
Kuntikana S, Bhat C, Kongot M, Bhat S, Kumar A (2016) An expeditious green cascade synthesis of 3-Arylideneaminoquinazolin-4(1H)-one derivatives via ‘solvent drop grinding’ and their antioxidant and dna protective studies. Chem Select 1:1723–1728. https://doi.org/10.1002/slct.201600362
Loupy A (2006) Microwaves in Organic Synthesis. Wiley-VCH
Martins MAP, Frizzo CP, Moreira DN, Buriol L, Machado P (2009) Solvent-Free Heterocyclic Synthesis. Chem Rev 109:4140–4182. https://doi.org/10.1021/cr9001098
Mhaske SB, Argade NP (2006) The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron 62:9787–9826. https://doi.org/10.1016/j.tet.2006.07.098
Michael JP (2005) Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep 22:627–646. https://doi.org/10.1039/B413750G
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Nielsen IB, Petersen MÅ, Lammich L, Nielsen MB, Andersen LH (2006) Absorption studies of neutral retinal schiff base chromophores. J Phys Chem A 110:12592–12596. https://doi.org/10.1021/jp064901r
Przybylski P, Huczynski A, Pyta K, Brzezinski B, Bartl F (2009) Biological properties of schiff bases and azo derivatives of phenols. Curr Org Chem 13:124–148. https://doi.org/10.2174/138527209787193774
Rakesh KP, Manukumar HM, Channe Gowda D (2015) Schiff’s bases of quinazolinone derivatives: synthesis and SAR studies of a novel series of potential anti-inflammatory and antioxidants. Bioorg Med Chem Lett 25:1072–1077. https://doi.org/10.1016/j.bmcl.2015.01.010
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Sawant VA, Yamgar BA, Sawant SK, Chavan SS (2009) Synthesis, structural characterization, thermal and electrochemical studies of mixed ligand Cu(II) complexes containing 2-phenyl-3-(benzylamino)-1,2-dihydroquinazoline-4-(3H)-one and bidentate N-donor ligands. Spectrochem Acta Part A 74:1100–1106. https://doi.org/10.1016/j.saa.2009.09.015
Schiff H (1864) Mittheilungen aus dem Universitätslaboratorium in Pisa: eine neue reihe organischer Basen. Justus Liebigs Annalen der Chemie 131:118–119. https://doi.org/10.1002/jlac.18641310113
Sztanke K, Maziarka A, Osinka A, Sztanke M (2013) An insight into synthetic Schiff bases revealing antiproliferative activities in vitro. Bioorg Med Chem 21:2648–3666. https://doi.org/10.1016/j.bmc.2013.04.037
Tian X, Schaich KM (2013) Effects of molecular structure on kinetics and dynamics of the trolox equivalent antioxidant capacity Assay with ABTS+•. J Agric Food Chem 61:5511–5519. https://doi.org/10.1021/jf4010725
Walker RB, Everette JD (2009) Comparative reaction rates of various antioxidants with ABTS radical cation. J Agric Food Chem 57:1156–1161. https://doi.org/10.1021/jf8026765
Wang XS, Sheng J, Lu L, Yang K, Li YL (2011) Combinatorial synthesis of 3-Arylidene aminoquinazolin-4(1H)-one derivatives catalyzed by iodine in ionic liquids. ACS Combinat Sci 13:196–199. https://doi.org/10.1021/co1000713
Zahedifard M, Faraj FL, Paydar M, Looi ChY, Hajrezaei M, Hasanpourghadi M, Kamalidehghan B, Majid NA, Ali HM, Abdulla MA (2015) Synthesis, characterization and apoptotic activity of quinazolinone Schiff base derivatives toward MCF-7 cells via intrinsic and extrinsic apoptosis pathways. Sci Rep 5(11544):1–17. https://doi.org/10.1038/srep11544
Zhang J, Cheng P, Ma Y, Liu J, Miao Z, Ren D, Fan C, Liang M, Liu L (2016) An efficient nano CuO-catalyzed synthesis and biological evaluation of quinazolinone Schiff base derivatives and bis-2,3-dihydroquinazolin-4(1H)-ones as potent antibacterial agents against Streptococcus lactis. Tetrahedron Lett 57:5271–5277. https://doi.org/10.1016/j.tetlet.2016.10.047
Zoubi WA (2013) Biological activities of Schiff bases and their complexes: a review of recent works. Int J Org Chem 3:73–95. https://doi.org/10.4236/ijoc.2013.33A008
Acknowledgements
The authors would like to acknowledge financial support from the Slovak Grant Agency, VEGA Grants No. 2/0100/14, 1/0041/15, 2/0027/16, 2/0022/18 and SP Grant 2003SP200280203 supported by the Research & Development Operational Program funded by the ERDF.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hricovíniová, Z., Hricovíni, M. & Kozics, K. New series of quinazolinone derived Schiff’s bases: synthesis, spectroscopic properties and evaluation of their antioxidant and cytotoxic activity. Chem. Pap. 72, 1041–1053 (2018). https://doi.org/10.1007/s11696-017-0345-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-017-0345-y