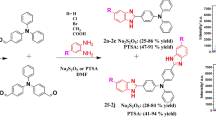
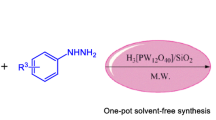
Abstract
A solvent-free microwave-assisted four-component synthesis of 1,2,4,5-tetrasubstituted imidazoles bearing a 4-aminophenyl substituent was studied by condensation of p-phenylenediamine, aryl diketone, benzaldehyde derivatives and ammonium acetate in the presence of solid support silica gel and catalyst Keggin-H3[PW12O40]. The effects of four components molar ratio along with catalyst loading, irradiation time on the yields were investigated. Also, the structures of synthesized compounds were characterized by FT-IR, HRMS, 1H NMR and 13C NMR spectroscopy. Furthermore, their ultraviolet–visible maximum absorption, liquid fluorescence emission maximum and quantum yields were, respectively, measured in 0.05 M H2SO4 aqueous solution and in dichloromethane. Simultaneously, solid fluorescence spectra were determined in the powder state. The relationships between the optical behavior and the polarity of the solvents for some compounds were assessed. The results showed that the fluorescence quantum efficiency was increased by introducing amino phenyl in comparison with benzyl on 1-position of trisubstitued imidazoles. The compounds synthesized were sensitive to the polarity of the solvents.



Similar content being viewed by others
References
Baghbanzadeh M, Carbone L, Cozzoli PD, Kappe CO (2011) Microwave-assisted synthesis of colloidal inorganic nanocrystals. Angew Chem Int Ed 50:2–50. doi:10.1002/anie.201101274
Balalaie S, Arabanian A (2000) One-pot synthesis of tetrasubstituted imidazoles catalyzed by zeolite HY and silica gel under microwave irradiation. Green Chem 2:274–276. doi:10.1039/B006201O
Balalaie S, Hashemi MM, Akhbari M (2003) A novel one-pot synthesis of tetrasubstituted imidazoles under solvent-free conditions and microwave irradiation. Tetrahedron Lett 44:1709–1711. doi:10.1016/S0040-4039(03)00018-2
Bhoi MN, Borada MA, Pithawalab EA, Patel HD (2016) Novel benzothiazole containing 4H-pyrimido[2,1-b]benzothiazoles derivatives: one pot, solvent-free microwave assisted synthesis and their biological evaluation. Arab J Chem. doi:10.1016/j.arabjc.2016.01.012
Buttke K, Baumgärtel H, Niclas H-J, Schneider M (1997) Synthese, Struktur und photophysikalische Eigenschaften von polyarylierten Imidazolen und Oxazolen. J Für Prakt Chem 339:721–728. doi:10.1002/prac.199733901131
Chandra AK, Turro NJ, Lyons AL, Stone P (1978) The intramolecular external heavy atom effect in bromo-, benzo-, and naphthonorbornenes. J Am Chem Soc 100:4964–4968. doi:10.1021/ja00484a008
Chen XT, Tong AJ (2014) Halogenated salicylaldehyde azines: the heavy atom effect on aggregation-induced emission enhancement properties. J Lumin 145:737–740. doi:10.1016/j.jlumin.2013.08.051
Chen GZh, Huang XZh, Zhang ZhZ, Xu JG, Wang JB (1990) Fluorescence analysis, 2nd edn. Science Press, Beijing, p 43
Chen Y, Gu Q, Chen Q, Chen XD, Zhang YM (2013) Efficient synthesis of 1-R1-2-R-4,5-di(furan-2-yl)-1H–imidazoles and their luminescence properties. Comptes Rend Chim 16:1103–1110. doi:10.1016/j.crci.2013.05.014
Chhanda M, Pradip KT (2012) A facile and efficient synthesis of tri- and tetrasubstituted imidazoles with potassium hydrogen sulfate and DB18C6 in an aqueous medium. Green Chem Lett Rev 5:109–120. doi:10.1080/17518253.2011.584572
Ci YX, Jia X (1986) Determination of relative fluorescence quantum yields using a simplified method. Chin J Anal Chem 14:616–618
Fletcher AN (1969) Quinine sulfate as a fluorescence quantum yield standard. Photochem Photobiol 9:439–444. doi:10.1111/j.1751-1097.1969.tb07311.x
Gao DW, Yu HF, Jia JL, Hua SY, Chen XD (1998) The synthesis of Furil. Acta Scient Nat Univ Jilinensis 1:107–109
Gill JE (1969) The fluorescence excitation spectrum of quinine bisulfate. Photochem Photobiol 9:313–322. doi:10.1111/j.1751-1097.1969.tb07295.x
Heravi MM, Bakhtiari K, Oskooie HA Taheri S et al (2007a) Synthesis of 2,4,5-triaryl-imidazoles catalyzed by NiCl2·6H2O under heterogeneous system. J Mol Catal A Chem 263(1):279–281. doi:10.1016/j.molcata.2006.08.070
Heravi MM, Derikvand F, Bamoharram FF (2007b) Highly efficient, four-component one-pot synthesis of tetrasubstituted imidazoles using Keggin-type heteropolyacid as green and reusable catalysts. J Mol Catal A-Chem 263:112–114. doi:10.1016/j.molcata.2006.08.048
Heung K, Urban MW (1999) Effect of discharge gases on microwave plasma reactions of imidazole on poly(dimethylsiloxane) surfaces: quantitative ATR FT-IR spectroscopic analysis. Langmuir 15:3499–3505. doi:10.1021/la970039q
Ho TI, Elangovan A, Hsu HY, Yang SW (2005) Highly Fluorescent N, N-dimethylaminophenylethynylarenes: synthesis, photophysical properties, and electrochemiluminescence. J Phys Chem B 109:8626–8633. doi:10.1021/jp0444518
Huang CG, Zhu XZ, Jin T, Tang YH (eds) (2012) Modern instrumental analysis. Beijing Chemical Industry Press, Beijing, p 85
Jaberi ZK, Barekat M (2010) One-pot synthesis of tri- and tetra-substituted imidazoles using sodium dihydrogen phosphate under solvent-free conditions. Chin Chem Lett 21:1183–1186. doi:10.1016/j.cclet.2010.06.012
Javid A, Heravi MM, Bamoharram FF, Nikpour M (2011) One-pot synthesis of tetrasubstituted imidazoles catalyzed by preyssler-type heteropolyacid. E J Chem 2:547–552. doi:10.1155/2011/980546
Jin QH, Dai SH, Huang KM (1999) Microwave Chemistry, 1st edn. Science Press, Beijing, pp 120–146
Karimi AR, Alimohammadi Z, Azizian J, Mohammadi AA, Mohammadizadeh MR (2006) Solvent-free synthesis of tetrasubstituted imidazoles on silica gel/NaHSO4 support. Catal Commun 7:728–732. doi:10.1016/j.catcom.2006.04.004
Kidwai M, Mothsra P (2006) A one-pot synthesis of 1,2,4,5-tetraarylimidazoles using molecular iodine as an efficient catalyst. Tetrahedron Lett 47:5029–5031. doi:10.1016/j.tetlet.2006.05.097
Kimura M, Shi K, Hashimoto KJ, Hu ZZ (2007) Development of functional imidazole derivatives: the influence of alkaline metal cations on the rates of CL reactions of 2-(phenyl and 4-dimethylaminophenyl-4-hydroperoxy-4-3′,4′-(15-crown-5)phenyl-5-3″-4″-(15-crown-5)phenyl-4H-isoimidazoles. Luminescence 22:229–235. doi:10.1002/bio.954
Kulhánek J, Bureš F (2012) Imidazole as a parent π-conjugated backbone in charge-transfer chromophores. Beilsten J Org Chem 8:25–49. doi:10.3762/bjoc.8.4
Li BZ, Gu Q, He YH, Zhao TQ, Wang SJ, Zhang YM (2012) Facile synthesis of trisubstituted imidazoles from 1,2-di(furan-2-yl)-2-oxoethyl carboxylates and their chemiluminescence. Comptes Rend Chim 9:784–792. doi:10.1016/j.crci.2012.06.005
Liu HN, Bara JE, Turner CH (2014) Tuning the adsorption interactions of imidazole derivatives with specific metal cations. J Phys Chem A 118:3944–3951. doi:10.1021/jp502222z
Mohammadi A, Keshvari H, Sandaroos R, Maleki B, Rouhi H, Moradi H, Sepehr Z, Damavandi S (2012) A highly efficient and reusable heterogeneous catalyst for the one-pot synthesis of tetrasubstituted imidazoles. Appl Catal A Gen 429–430:73–78. doi:10.1016/j.apcata.2012.04.011
Murthy SN, Madhav B, Nageswar YVD (2010) DABCO as a mild and efficient catalytic system for the synthesis of highly substituted imidazoles via multi-component condensation strategy. Tetrahedron Lett 51:5252–5257. doi:10.1016/j.tetlet.2010.07.128
Na K, Lee ES, Bae YH (2007) Self-organized nanogels responding to tumor extracellular pH:pH-dependent drug release and in vitro cytotoxicity against MCF-7 cells. Bioconjug Chem 18:1568–1574. doi:10.1021/bc070052e
Nagarapu L, Apuri S, Kantevari S (2007) Potassium dodecatugstocobaltate trihydrate (K5CoW12O40·3H2O): a mild and efficient reusable catalyst for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles under conventional heating and microwave irradiation. J Mol Catal A Chem 266:104–108. doi:10.1016/j.molcata.2006.10.056
Nasr-Esfahani M, Montazerozohori M, Abdizadeh T (2015) Multi-component synthesis of highly substituted imidazoles catalyzed by nanorod vanadatesulfuric acid. Chem Pap 69:1491–1499. doi:10.1515/chempap-2015-0156
Shirai K, Matsuoka M, Fukunishi K (2000) New syntheses and solid state fluorescence of azomethine dyes derived from diaminomaleonitrile and 2,5-diamino-3,6-dicyanopyrazine. Dyes Pigment 47:107–115. doi:10.1016/S0143-7208(00)00068-1
Siddiqui SA, Narkhede UC, Palimkar SS, Daniel T, Lahoti RJ, Srinivasan KV (2005) Room temperature ionic liquid promoted improved and rapid synthesis of 2,4,5-triaryl imidazoles from aryl aldehydes and 1,2-diketones or α-hydroxyketone. Tetrahedron 61:3539–3546. doi:10.1016/j.tet.2005.01.116
Smith AL, DeMorin FF, Paras NA, Huang Q, Petkus JK, Doherty EM (2009) Selective inhibitors of the mutant B-Raf pathway: discovery of a potent and orally bioavailable aminoisoquinoline. J Med Chem 52:6189–6192. doi:10.1021/jm901081g
Subhasis S, Ganesh CN et al (2009) L-Proline:an efficient catalyst for the one-pot M.synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles. Tetrahedron 65:10155–10161. doi:10.1016/j.tet.2009.10.019
Tian M, Wang Ch, Wang LG, Luo K, Zhao A, Guo CC (2014) Study on the synthesis and structure-effect relationship of multi-aryl imidazoles with their fluorescence properties. Luminescence 29:540–548. doi:10.1002/bio.2580
Wagner GK, Kotschenreuther D, Zimmermann W, Laufer SA (2003) Identification of regioisomers in a series of N-substituted Pyridin-4-yl Imidazole derivatives by regiospecific synthesis, GC/MS, and 1H NMR. J Org Chem 68:4527–4530. doi:10.1021/jo026619w
Xu JG, Wang ZB (2006) Fluorimetry, 3ed. Science Press. p, Beijing, p 27
Zhang J, Zhao T-Q, Chen Y, Chen X-D, Chang H-K, Zhang Y-M, Hua Sh-Ch (2015) Microwave-assisted solvent-free synthesis and luminescence properties of 2-substituted-4,5-di(2-furyl)-1H-imidazoles. Chem Pap 69:325–338. doi:10.1515/chempap-2015-0014
Zhang WX, Dong XC, Zhao WL (2016) Microwave-assisted solventless reaction of iridium-catalyzed alkylation of amines with alcohols in the absence of base. Org Lett 13(19):5386–5389. doi:10.1021/ol202281h
Acknowledgements
We are grateful to Mr. Ch. Y. Wang for the NMR spectra and Mr. Zh. L. Wei for the MS spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, Lq., Chen, Y., Sun, Xf. et al. Microwave-assisted solvent-free catalyzed synthesis and luminescence properties of 1,2,4,5-tetrasubstituted imidazoles bearing a 4-aminophenyl substituent. Chem. Pap. 71, 627–637 (2017). https://doi.org/10.1007/s11696-016-0051-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-016-0051-1