Abstract
Introduction
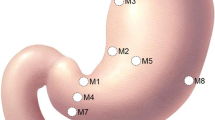
Visible light spectroscopy (VLS) represents a sensitive, non-invasive method to quantify tissue oxygen levels and detect hypoxemia. The aim of this study was to assess the microperfusion patterns of the gastric pouch during laparoscopic Roux-en-Y gastric bypass (LRYGB) using the VLS technique.
Methods
Twenty patients were enrolled. Tissue oxygenation (StO2%) measurements were performed at three different localizations of the gastric wall, prior and after the creation of the gastric pouch, and after the creation of the gastro-jejunostomy.
Results
Prior to the creation of the gastric pouch, the lowest StO2% levels were observed at the level of the distal esophagus with a median StO2% of 43 (IQR 40.8–49.5). After the creation of the gastric pouch and after the creation of the gastro-jejunostomy, the lowest StO2% levels were recorded at the level of the His angle with median values of 29% (IQR 20–38.5) and 34.5% (IQR 19–39), respectively. The highest mean StO2 reduction was recorded at the level of the His angle after the creation of the gastric pouch, and it was 18.3% (SD ± 18.1%, p < 0.001). A reduction of StO2% was recorded at all localizations after the formation of the gastro-jejunostomy compared to the beginning of the operation, but the mean differences of the StO2% levels were statistically significant only at the resection line of the pouch and at the His angle (p = 0.044 and p < 0.001, respectively).
Conclusion
Gastric pouch demonstrates reduction of StO2% during LRYGB. VLS is a useful technique to assess microperfusion patterns of the stomach during LRYGB.
Graphical abstract








Similar content being viewed by others
References
Welbourn R, Hollyman M, Kinsman R, et al. Bariatric surgery worldwide: baseline demographic description and one-year outcomes from the fourth IFSO global registry report 2018. Obes Surg. 2019;29(3):782–95. https://doi.org/10.1007/s11695-018-3593-1.
Wang MC, Guo XH, Zhang YW, et al. Laparoscopic Roux-en-Y gastric bypass versus sleeve gastrectomy for obese patients with Type 2 diabetes: a meta-analysis of randomized controlled trials. Am Surg. 2015;81(2):166–71. https://doi.org/10.1177/000313481508100229.
Peterli R, Wolnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass onweight loss in patients with morbid obesity the sm-boss randomized clinical trial. JAMA - J Am Med Assoc. 2018;319(3):255–65. https://doi.org/10.1001/jama.2017.20897.
Vidarsson B, Sundbom M, Edholm D. Incidence and treatment of leak at the gastrojejunostomy in Roux-en-Y gastric bypass: a cohort study of 40,844 patients. Surg Obes Relat Dis. 2019;15(7):1075–9. https://doi.org/10.1016/j.soard.2019.04.033.
Smith MD, Adeniji A, Wahed AS, et al. Technical factors associated with anastomotic leak after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2015;11(2):313–20. https://doi.org/10.1016/j.soard.2014.05.036.
Thompson SK, Chang EY, Jobe BA. Clinical review: healing in gastrointestinal anastomoses, part I. Microsurgery. 2006;26(3):131–6. https://doi.org/10.1002/micr.20197.
Sverdén E, Mattsson F, Sondén A, et al. Risk factors for marginal ulcer after gastric bypass surgery for obesity: a population-based cohort study. Ann Surg. 2016;263(4):733–7. https://doi.org/10.1097/SLA.0000000000001300.
El-Hayek K, Timratana P, Shimizu H, et al. Marginal ulcer after Roux-en-Y gastric bypass: what have we really learned? Surg Endosc. 2012;26(10):2789–96. https://doi.org/10.1007/s00464-012-2280-x.
Wilson JA, Romagnuolo J, Byrne TK, et al. Predictors of endoscopic findings after Roux-en-Y gastric bypass. Am J Gastroenterol. 2006;101(10):2194–9. https://doi.org/10.1111/j.1572-0241.2006.00770.x.
Benaron DA, Parachikov IH, Friedland S, et al. Continuous, noninvasive, and localized microvascular tissue oximetry using visible light spectroscopy. Anesthesiology. 2004;25(6):1469–75. https://doi.org/10.1097/00000542-200406000-00019.
Karliczek A, Benaron DA, Baas PC, et al. Intraoperative assessment of microperfusion with visible light spectroscopy for prediction of anastomotic leakage in colorectal anastomoses. Colorectal Dis. 2010;12(10):1018–25. https://doi.org/10.1111/j.1463-1318.2009.01944.x.
Karliczek A, Benaron DA, Baas PC, et al. Intraoperative assessment of microperfusion with visible light spectroscopy in esophageal and colorectal anastomoses. Eur Surg Res. 2008;41(3):303–11. https://doi.org/10.1159/000155880.
Delko T, Hoffmann H, Kraljevic M, et al. Intraoperative Patterns of gastric microperfusion during laparoscopic sleeve gastrectomy. Obes Surg. 2017;27(4):926–32. https://doi.org/10.1007/s11695-016-2386-7.
Saber AA, Azar N, Dekal M, et al. Computed tomographic scan mapping of gastric wall perfusion and clinical implications. Am J Surg. 2015;209(6):999–1006. https://doi.org/10.1016/j.amjsurg.2014.05.023.
Prudius V, Procházka V, Pavlovský Z, et al. Vascular anatomy of the stomach related to resection procedures strategy. Surg Radiol Anat. 2017;39(4):433–40. https://doi.org/10.1007/s00276-016-1746-2.
Varban OA, Cassidy RB, Sheetz KH, et al. Technique or technology? Evaluating leaks after gastric bypass. Surg Obes Relat Dis. 2016;12(2):264–72. https://doi.org/10.1016/j.soard.2015.07.013.
Acquafresca PA, Palermo M, Rogula T, et al. Early surgical complications after gastric by-pass: a literature review. Arq Bras Cir Dig. 2015;28(1):74–80. https://doi.org/10.1590/S0102-67202015000100019.
Fernandez AZ, DeMaria EJ, Tichansky DS, et al. Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc Other Interv Tech. 2004;18(2):193–7. https://doi.org/10.1007/s00464-003-8926-y.
Pędziwiatr M, Małczak P, Wierdak M, et al. Revisional gastric bypass is inferior to primary gastric bypass in terms of short-and long-term outcomes—Systematic review and meta-analysis. Obes Surg. 2018;28(7):2083–91. https://doi.org/10.1007/s11695-018-3300-2.
Benois M, Sebastianelli L, Morisot A, et al. Revisional but not conversional gastric bypass surgery increases the risk of leaks: review of 176 redo out of 932 consecutive cases. Obes Surg. 2018;28(9):2903–11. https://doi.org/10.1007/s11695-018-3311-z.
Iannelli A, Schneck A-S, Hébuterne X, et al. Gastric pouch resizing for Roux-en-Y gastric bypass failure in patients with a dilated pouch. Surg Obes Relat Dis. 2013;9(2):260–7. https://doi.org/10.1016/j.soard.2012.05.003.
Borbély Y, Winkler C, Kröll D, et al. Pouch Reshaping for significant weight regain after Roux-en-y gastric bypass. Obes Surg. 2017;27(2):439–44. https://doi.org/10.1007/s11695-016-2329-3.
Shikora SA, Mahoney CB. Clinical benefit of gastric staple line reinforcement (SLR) in gastrointestinal surgery: a meta-analysis. Obes Surg. 2015;25(7):1133–41. https://doi.org/10.1007/s11695-015-1703-x.
Coblijn UK, Lagarde SM, de Castro SMM, et al. Symptomatic marginal ulcer disease after Roux-en-Y gastric bypass: incidence, risk factors and management. Obes Surg. 2015;25(5):805–11. https://doi.org/10.1007/s11695-014-1482-9.
Edholm D, Ottosson J, Sundbom M. Importance of pouch size in laparoscopic Roux-en-Y gastric bypass: a cohort study of 14,168 patients. Surg Endosc. 2016;30(5):2011–5. https://doi.org/10.1007/s00464-015-4432-2.
Dittrich L, Schwenninger MV, Dittrich K, et al. Marginal ulcers after laparoscopic Roux-en-Y gastric bypass: analysis of the amount of daily and lifetime smoking on postoperative risk. Surg Obes Relat Dis. 2020;16(3):389–96. https://doi.org/10.1016/j.soard.2019.11.022.
Francis N, Dort J, Cho E, et al. SAGES and EAES recommendations for minimally invasive surgery during COVID-19 pandemic. Surg Endosc. 2020. https://doi.org/10.1007/s00464-020-07565-w.
Spaniolas K, Yang J, Crowley S, et al. Association of long-term anastomotic ulceration after Roux-en-Y gastric bypass with tobacco smoking. JAMA Surg. 2018;153(9):862–4. https://doi.org/10.1001/jamasurg.2018.1616.
Süsstrunk J, Wartmann L, Mattiello D, et al. Incidence and prognostic factors for the development of symptomatic and asymptomatic marginal ulcers after Roux-en-Y gastric bypass procedures. Obes Surg. 2021;31(7):3005–14. https://doi.org/10.1007/s11695-021-05363-4.
Chahine E, Kassir R, Dirani M, et al. Surgical management of gastrogastric fistula after Roux-en-Y gastric bypass: 10-year experience. Obes Surg. 2018;28(4):939–44. https://doi.org/10.1007/s11695-017-2949-2.
Pauli EM, Beshir H, Mathew A. Gastrogastric fistulae following gastric bypass surgery—clinical recognition and treatment. Curr Gastroenterol Rep. 2014;16(9):1–8. https://doi.org/10.1007/s11894-014-0405-1.
Iossa A, Abdelgawad M, Watkins BM, et al. Leaks after laparoscopic sleeve gastrectomy: overview of pathogenesis and risk factors. Langenbeck’s Arch Surg. 2016;401(6):757–66. https://doi.org/10.1007/s00423-016-1464-6.
Funding
The study was funded with grants from “Stiftung für chirurgische Forschung und Spitalmanagement” and grants from “Freiwillige Akademische Gesellschaft Basel.” Ioannis I. Lazaridis reports grants from Department of Surgery, University Hospital Basel, from Freiwillige Akademische Gesellschaft Basel, from Nora van Meeuwen- Häfliger Foundation, outside the submitted work. Romano Schneider reports grants from University of Basel, grants from Department of Surgery, University Hospital Basel, grants from SFCS, grants from Freiwillige Akademische Gesellschaft Basel and grants from Gebauer Stiftung, outside the submitted work. Marko Kraljević reports grants from Berrie Scholar in Diabetes Research, outside the submitted work. Jennifer M Klasen reports grants from Department of Surgery, University Hospital Basel and from Group of Educational Affairs (WGEA), honoraria from BPER, Centre for Faculty Development and CERI, Centre for Education Research & Innovation, outside the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/ or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• During LRYGB, real-time tissue oxygenation measurements using VLS showed a significant reduction of tissue oxygenation at the ventral side of the staple line of the gastric pouch and at the level of the His angle.
• VLS during LRYGB is a safe and technically feasible procedure, which does not cause disturbance of the routine surgical workflow.
• Localized impairment of pouch microperfusion may be the pathophysiologic precursor of complications, such as anastomotic leaks, staple line leaks, gastro-gastric fistulas, and anastomotic ulcers.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lazaridis, I.I., Schneider, R., Stocker, R. et al. Intraoperative Patterns of Gastric Microperfusion During Laparoscopic Roux-en-Y Gastric Bypass. OBES SURG 32, 4047–4056 (2022). https://doi.org/10.1007/s11695-022-06318-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-022-06318-z