Abstract
Background
Metabolic/bariatric surgery is a highly effective treatment for obesity and metabolic diseases. Serum glucagon, bile acids, and FGF-19 are key effectors of various metabolic processes and may play central roles in bariatric surgical outcomes. It is unclear whether these factors behave similarly after Roux-en-Y gastric bypass (RYGB) vs vertical sleeve gastrectomy (VSG).
Methods
Serum glucagon, bile acids (cholic acid [CA], chenodeoxycholic acid [CDCA], deoxycholic acid [DCA]), and FGF-19 were analyzed in samples of fasting blood collected before bariatric surgery, on postoperative days 2 and 10, and at 3- and 6-month follow-up.
Results
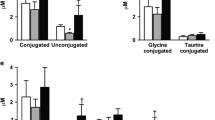
From September 2016 to July 2017, patients with obesity underwent RYGB or VSG; 42 patients (RYGB n = 21; VSG n = 21) were included in the analysis. In the RYGB group, glucagon, CA, and CDCA increased continuously after surgery (p = 0.0003, p = 0.0009, p = 0.0001, respectively); after an initial decrease (p = 0.04), DCA increased significantly (p = 0.0386). Serum FGF-19 was unchanged. In the VSG group, glucagon increased on day 2 (p = 0.0080), but decreased over the 6-month study course (p = 0.0025). Primary BAs (CA and CDCA) decreased immediately after surgery (p = 0.0016, p = 0.0091) and then rose (p = 0.0350, p = 0.0350); DCA followed the curve of the primary BAs until it fell off at 6 months (p = 0.0005). VSG group serum FGF-19 trended upward.
Conclusion
RYGB and VSG involve different surgical techniques and final anatomical configurations. Between postoperative day 2 and 6-month follow-up, RYGB and VSG resulted in divergent patterns of change in serum glucagon, bile acids, and FGF-19.
Graphical abstract





Similar content being viewed by others
References
Wadden TA, Bantle JP, Blackburn GL, Bolin P, Brancati FL, Bray GA, et al. Eight-year weight losses with an intensive lifestyle intervention: The look AHEAD study. Obesity. Hoboken: Wiley. 2014;22:5–13.
Sjostrom L, Narbro K, Sjostrom D, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med Waltham: Massachusetts Medical Soc. 2007;357:741–52.
Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab Washington: Endocrine Soc. 2008;93:S89–96.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes-5-year outcomes. N Engl J Med Waltham: Massachusetts Medical Soc. 2017;376:641–51.
Knop FK, Taylor R. Mechanism of metabolic advantages after bariatric surgery it’s all gastrointestinal factors versus it’s all food restriction. Diabetes Care Alexandria: Amer Diabetes Assoc. 2013;36:S287–91.
Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–42.
Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes. 2005;2010(34):462–71.
Bose M, Teixeira J, Olivan B, Bawa B, Arias S, Machineni S, et al. Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J Diabetes. 2010;2:47–55.
Morínigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–601.
Morínigo R, Moizé V, Musri M, Lacy AM, Navarro S, Marín JL, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–40.
Albaugh VL, Flynn CR, Cai S, Xiao Y, Tamboli RA, Abumrad NN. Early Increases in bile acids post roux-en-y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J Clin Endocrinol Metab Oxford Academic. 2015;100:E1225–33.
Hofmann AF, Hagey LR. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci Basel: Springer Basel Ag. 2008;65:2461–83.
Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov London: Nature Publishing Group. 2008;7:678–93.
Chiang JYL. Bile acid metabolism and signaling. Compr Physiol Hoboken: Wiley. 2013;3:1191–212.
Staels B, Fonseca VA. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care Am Diabetes Assoc. 2009;32:S237–45.
Mueller M, Thorell A, Claudel T, Jha P, Koefeler H, Lackner C, et al. Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol Amsterdam: Elsevier. 2015;62:1398–404.
Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–9.
Alexiadou K, Cuenco J, Howard J, Wewer Albrechtsen NJ, Ilesanmi I, Kamocka A, et al. Proglucagon peptide secretion profiles in type 2 diabetes before and after bariatric surgery: 1-year prospective study. BMJ Open Diabetes Res Care. 2020;8
Demant M, Bagger JI, Suppli MP, Lund A, Gyldenløve M, Hansen KB, et al. Determinants of fasting hyperglucagonemia in patients with type 2 diabetes and nondiabetic control subjects. Metab Syndr Relat Disord. 2018;16:530–6.
Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–16.
Purnell JQ, Johnson GS, Wahed AS, Dalla Man C, Piccinini F, Cobelli C, et al. Prospective evaluation of insulin and incretin dynamics in obese adults with and without diabetes for 2 years after Roux-en-Y gastric bypass. Diabetologia. 2018;61:1142–54.
Jacobsen SH, Olesen SC, Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22:1084–96.
Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KDR, et al. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity. Hoboken: Wiley. 2014;22:390–400.
Cummings BP, Bettaieb A, Graham JL, Stanhope KL, Kowala M, Haj FG, et al. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153:3620–32.
Ding L, Sousa KM, Jin L, Dong B, Kim B-W, Ramirez R, et al. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatol Baltim Md. 2016;64:760–73.
Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. London: Nature Publishing Group; 2014;509:183-+.
Bhutta HY, Rajpal N, White W, Freudenberg JM, Liu Y, Way J, et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PloS One. 2015;10:e0122273.
Spinelli V, Lalloyer F, Baud G, Osto E, Kouach M, Daoudi M, et al. Influence of Roux-en-Y gastric bypass on plasma bile acid profiles: a comparative study between rats, pigs and humans. Int J Obes. 2005;2016(40):1260–7.
Khan FH, Shaw L, Zhang W, Salazar Gonzalez RM, Mowery S, Oehrle M, et al. Fibroblast growth factor 21 correlates with weight loss after vertical sleeve gastrectomy in adolescents. Obes Silver Spring Md. 2016;24:2377–83.
Jahansouz C, Xu H, Hertzel AV, Serrot FJ, Kvalheim N, Cole A, et al. Bile acids increase independently from hypocaloric restriction after bariatric surgery. Ann Surg. 2016;264:1022–8.
Escalona A, Munoz R, Irribarra V, Solari S, Allende F, Francisco MJ. Bile acids synthesis decreases after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis, vol. 12. New York: Elsevier Science Inc; 2016. p. 763–9.
Belgaumkar AP, Vincent RP, Carswell KA, Hughes RD, Alaghband-Zadeh J, Mitry RR, et al. Changes in bile acid profile after laparoscopic sleeve gastrectomy are associated with improvements in metabolic profile and fatty liver disease. Obes Surg. 2016;26:1195–202.
Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T, et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity. 2013;21:E660–8.
Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high–molecular weight adiponectin levels are increased after bariatric surgery. Metab - Clin Exp Elsevier. 2009;58:1400–7.
Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, et al. A Role for Fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care Am Diabetes Assoc. 2013;36:1859–64.
Jansen PLM, van Werven J, Aarts E, Berends F, Janssen I, Stoker J, et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis Basel Switz. 2011;29:48–51.
Haluzíková D, Lacinová Z, Kaválková P, Drápalová J, Křížová J, Bártlová M, et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obes Silver Spring Md. 2013;21:1335–42.
Albaugh VL, Banan B, Ajouz H, Abumrad NN, Flynn CR. Bile acids and bariatric surgery. Mol Aspects Med Amsterdam: Elsevier. 2017;56:75–89.
Ferrannini E, Camastra S, Astiarraga B, Nannipieri M, Castro-Perez J, Xie D, et al. Increased bile acid synthesis and deconjugation after biliopancreatic diversion. diabetes. Alexandria: Amer Diabetes Assoc. 2015;64:3377–85.
De Vuono S, Ricci MA, Nulli Migliola E, Monti MC, Morretta E, Boni M, et al. Serum bile acid levels before and after sleeve gastrectomy and their correlation with obesity-related comorbidities. Obes Surg. 2019;29:2517–26.
Trung VN, Yamamoto H, Furukawa A, Yamaguchi T, Murata S, Yoshimura M, et al. Enhanced intestinal motility during oral glucose tolerance test after laparoscopic sleeve gastrectomy: preliminary results using cine magnetic resonance imaging. Plos One Public Library Sci. 2013;8:e65739.
Einarsson C, Hillebrant CG, Axelson M. Effects of treatment with deoxycholic acid and chenodeoxycholic acid on the hepatic synthesis of cholesterol and bile acids in healthy subjects. Hepatol Baltim Md. 2001;33:1189–93.
Zhang C, Zhang J, Zhou Z. Changes in fasting bile acid profiles after Roux-en-Y gastric bypass and sleeve gastrectomy. Medicine (Baltimore). 2021;100:e23939.
Holst JJ, Madsbad S, Bojsen-Møller KN, Svane MS, Jørgensen NB, Dirksen C, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2018;14:708–14.
Yang J, Gao Z, Williams DB, Wang C, Lee S, Zhou X, et al. Effect of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy on fasting gastrointestinal and pancreatic peptide hormones: a prospective nonrandomized trial. Surg Obes Relat Dis Off J Am Soc Bariatr Surg. 2018;14:1521–9.
Chiang JYL. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–66.
Li S, Hsu DDF, Li B, Luo X, Alderson N, Qiao L, et al. Cytoplasmic tyrosine phosphatase Shp2 coordinates hepatic regulation of bile acid and FGF15/19 signaling to repress bile acid synthesis. Cell Metab. 2014;20:320–32.
Acknowledgements
We thank TW McGlennon, McGlennon MotiMetrics, Maiden Rock, WI, USA, for statistical analysis review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All procedures of the study are ethically compliant and were approved (EK 416092015) by the Institutional Review Board of University Medicine Mannheim, Germany. The study was performed in accord with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments.
Consent to Participate
Written informed consent was obtained from all study participants.
Conflict of Interest
The authors declare that they have no conflicts of interest or financial ties to disclose. JN Buchwald received a small grant for manuscript development.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
1. In the RYGB group: Glucagon and the primary bile acids CA and CDCA increased continuously after surgery, while the secondary bile acid DCA decreased initially and later significantly increased.
2. In the VSG group: Glucagon increased initially but later decreased. The primary bile acids CA and CDCA, as well as DCA, decreased immediately and showed no significant change thereafter.
3. RYGB and VSG resulted in divergent patterns of change in serum glucagon, bile acids, and FGF-19. The different alterations in bile acid levels following RYGB and VSG have been confirmed by a recent meta-analysis.
Rights and permissions
About this article
Cite this article
Yang, C., Brecht, J., Weiß, C. et al. Serum Glucagon, Bile Acids, and FGF-19: Metabolic Behavior Patterns After Roux-en-Y Gastric Bypass and Vertical Sleeve Gastrectomy. OBES SURG 31, 4939–4946 (2021). https://doi.org/10.1007/s11695-021-05677-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05677-3