Abstract
Purpose
Obesity is one of the most important risk factors for acute pancreatitis. Based on the effect of sleeve gastrectomy (SG) on improving body weight and blood lipids, we investigated whether SG is beneficial in improving pancreatitis in obese rats.
Materials and Methods
Two studies were used to evaluate the effect of SG on the first onset of pancreatitis and acute episodes of recurrent pancreatitis in obese rats. A high-fat diet (HFD) for 8 weeks resulted in obesity in rats. Study 1: Obese rats were treated with SG and sham surgery. Pancreatitis was induced by intraperitoneal injection of cerulein at 6 weeks after surgery. The severity of pancreatitis was assessed by histological examination, cytokines, and infiltration of inflammatory cells. Study 2 performed the same procedure as in study 1, except that rats were intraperitoneally injected with a small dose of cerulein three times a week for 6 weeks before surgery to induce recurrent pancreatitis.
Results
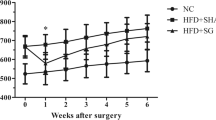
The body weight, food intake, and blood lipids of SG rats in study 1 and study 2 were significantly lower than those of sham rats during the 6 weeks after surgery. Compared with sham rats, SG rats in both studies had fewer inflammatory cytokines, inflammatory cell infiltration, and pathological injury in the pancreas after cerulein-induced acute pancreatitis.
Conclusion
SG reduces the severity of the first onset of pancreatitis and the seriousness of acute episodes of recurrent pancreatitis. The improvement of lipid metabolism and body weight by SG may play an important role in this effect.
Graphical abstract








Similar content being viewed by others
References
Khatua B, El-Kurdi B, Singh VP. Obesity and pancreatitis. Curr Opin Gastroenterol. 2017;33(5):374–82.
Hong YP, Yu J. High-fat diet aggravates acute pancreatitis via TLR4-mediated necroptosis and inflammation in rats. Oxidative Med Cell Longev. 2020;2020:8172714. Epub 2020 Jan 8
Yashima Y, Isayama H, Tsujino T, et al. A large volume of visceral adipose tissue leads to severe acute pancreatitis. J Gastroenterol. 2011;46(10):1213–8.
Hansen SEJ, Madsen CM, Varbo A, et al. Body mass index, triglycerides, and risk of acute pancreatitis: a population-based study of 118 000 Individuals. J Clin Endocrinol Metab. 2020;105(1):dgz059.
Radmard AR, Merat S, Kooraki S, et al. Gallstone disease and obesity: a population-based study on abdominal fat distribution and gender differences. Ann Hepatol. 2015;14(5):702–9.
Piťha J, Kovář J, Blahová T. Fasting and nonfasting triglycerides in cardiovascular and other diseases. Physiol Res. 2015;64(Suppl 3):S323–30.
Navina S, Acharya C, DeLany JP, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3(107):107ra10.
Spiegel HU, Skawran S. From longitudinal gastric resection to sleeve gastrectomy--revival of a previously established surgical procedure. J Gastrointest Surg. 2011;15(1):219–28.
English WJ, DeMaria EJ, Brethauer SA, et al. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;14(3):259–63.
Kawano Y, Ohta M, Hirashita T, et al. Effects of sleeve gastrectomy on lipid metabolism in an obese diabetic rat model. Obes Surg. 2013;23(12):1947–56.
Watanabe K, Ohta M, Takayama H, et al. Effects of sleeve gastrectomy on nonalcoholic fatty liver disease in an obese rat model. Obes Surg. 2018;28(6):1532–9.
Song Y, Deng H, Zhou J, et al. The effects of laparoscopic sleeve gastrectomy on obesity-related hypertriglyceridemia-induced acute pancreatitis. Obes Surg. 2018;28(12):3872–9.
Rong Y, Ren J, Song W, Xiang R, Ge Y, Lu W, et al. Resveratrol suppresses severe acute pancreatitis-induced microcirculation disturbance through targeting SIRT1-FOXO1 Axis. Oxidative Med Cell Longev. 2021;2021:8891544.
Yamaguchi H, Weidenbach H, Lührs H, et al. Combined treatment with C1 esterase inhibitor and antithrombin III improves survival in severe acute experimental pancreatitis. Gut. 1997;40(4):531–5.
Pan Y, Li Y, Gao L, et al. Development of a novel model of hypertriglyceridemic acute pancreatitis in mice. Sci Rep. 2017;7:40799.
Pezzilli R, Miniero R, Cappelletti O, et al. Serum interleukin 6 in the prognosis of acute biliary pancreatitis. Ital J Gastroenterol Hepatol. 1998;30(3):291–4.
Bhatia M, Neoptolemos JP, Slavin J. Inflammatory mediators as therapeutic targets in acute pancreatitis. Curr Opin Investig Drugs. 2001;2(4):496–501.
Denham W, Yang J, Fink G, et al. Gene targeting demonstrates additive detrimental effects of interleukin 1 and tumor necrosis factor during pancreatitis. Gastroenterology. 1997;113(5):1741–6.
Bhatia M, Wong FL, Cao Y, et al. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5(2-3):132–44.
Manohar M, Verma AK, Venkateshaiah SU, et al. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther. 2017;8(1):10–25.
Awla D, Abdulla A, Zhang S, et al. Lymphocyte function antigen-1 regulates neutrophil recruitment and tissue damage in acute pancreatitis. Br J Pharmacol. 2011;163(2):413–23.
Abdulla A, Awla D, Thorlacius H, et al. Role of neutrophils in the activation of trypsinogen in severe acute pancreatitis. J Leukoc Biol. 2011;90(5):975–82.
Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–47. Epub 2019/09/19
Chang YT, Chang MC, Tung CC, et al. Distinctive roles of unsaturated and saturated fatty acids in hyperlipidemic pancreatitis. World J Gastroenterol. 2015;21(32):9534–43.
Jeong YK, Kim H. A mini-review on the effect of docosahexaenoic acid (DHA) on cerulein-induced and hypertriglyceridemic acute pancreatitis. Int J Mol Sci. 2017;18(11):2239.
Hussan H, Ugbarugba E, Porter K, et al. The type of bariatric surgery impacts the risk of acute pancreatitis: a nationwide study. Clin Transl Gastroenterol. 2018;9(9):179.
Hsu S-Y, Ser K-H, Chong K, et al. Mini-gastric bypass surgery for hypertriglyceridemia-induced pancreatitis. Formos J Surg. 2012;45(6):187–90.
Ren Y, He M, Xian Y, et al. RYGB in treating patients with obesity, hypertriglyceridemia-induced acute pancreatitis, and diabetes: kill three birds with one stone? Obes Surg. 2020;30(5):2033–5.
Kröner PT, Simons-Linares CR. Acute pancreatitis in patients with a history of bariatric surgery: is it less severe? Obes Surg. 2020;30(6):2325–30.
Acknowledgements
We thank John T. Cathey for English language assistance (Peerwith, John Cathey, https://goo.gl/MSJTA7).
Funding
This study was supported by the Foundation of Sichuan Health Committee (18PJ496).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Informed Consent
Informed Consent does not apply.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
1. SG significantly improved body weight and lipid metabolism in obese rats.
2. SG reduces the severity of the first onset of pancreatitis in obese rats.
3. SG reduces the severity of acute episode of recurrent pancreatitis in obese rats.
Rights and permissions
About this article
Cite this article
Xian, Y., Wu, Y., He, M. et al. Sleeve Gastrectomy Attenuates the Severity of Cerulein-Induced Acute Pancreatitis in Obese Rats. OBES SURG 31, 4107–4117 (2021). https://doi.org/10.1007/s11695-021-05521-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05521-8