Abstract
Purpose
Neuromodulation, such as vagal nerve stimulation and intestinal electrical stimulation, has been introduced for the treatment of obesity and diabetes. Ideally, neuromodulation should be applied automatically after food intake. The purpose of this study was to develop a method of automatic food intake detection through dynamic analysis of heart rate variability (HRV).
Materials and Methods
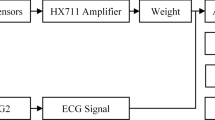
Two experiments were conducted: (1) a small sample series with a standard test meal and (2) a large sample series with varying meal size. Electrocardiograms (ECGs) were collected in the fasting and postprandial states. Each ECG was processed to compute the HRV. For each HRV segment, time- and frequency-domain features were derived and used as inputs to train and test an artificial neural network (ANN). The ANN was trained and tested with different cross-validation methods.
Results
The highest classification accuracy reached with leave-one-subject-out-leave-one-sample-out cross-validation was 0.93 in experiment 1 and 0.88 in experiment 2. Retraining the ANN on recordings of a subject drastically increased the achieved accuracy for that subject to values of 0.995 and 0.95 in experiments 1 and 2, respectively.
Conclusions
Automatic food intake detection by ANNs, using features from the HRV, is feasible and may have a great potential for neuromodulation-based treatments of meal-related disorders.





Similar content being viewed by others
Abbreviations
- ANN:
-
Artificial neural networks
- FGIDs:
-
Functional gastrointestinal disorders
- HRV:
-
Heart rate variability
References
Sallam HS, Chen JDZ. Colon electrical stimulation: potential use for treatment of obesity. Obesity. 2011;19(9):1761–7.
Arrone LJ. Epidemiology, morbidity, and treatment of overweight and obesity. J Clin Psychiatry. 2001;62(23):13–22.
Spieker EA, Pyzocha N. Economic impact of obesity. Prim Care. 2016;43:83–95.
Department of Health and Human Services. Atlanta: Centers for Disease Control and Prevention; c1995-2019 [cited 2018 Aug 2]. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. (Accessed 2 Aug 2018).
American Diabetes Association. Complication; c1995-2019 [cited 2018 Aug 2]. Available from: http://diabetes.org/living-with-diabetes/complications/.
Parkman HP, Doma S. Importance of gastrointestinal motility disorders. Pract Gastroenterol. 2006;30(9):23–40.
Talley N. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil. 2008;20(1):121–9.
Cordera R, Adami GF. From bariatric to metabolic surgery: looking for a “disease modifier” surgery for type 2 diabetes. World J Diabetes. 2016;7(2):27–33.
Department of Health and Human Services, USA. Insulin, medicines, & other diabetes treatments. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases. c1995-2019 [cited 2018 Aug 2]. Available from: https://www.niddk.nih.gov/health-information/diabetes/overview/insulin-medicines-treatments
Huertas-Ceballos A, Macarthur C, Logan S. Dietary interventions for recurrent abdominal pain (RAP) in childhood. Cochrane Database Syst Rev. 2002:CD003019.
Lebenthal E, Rossi TM, Nord KS, et al. Recurrent abdominal pain and lactose absorption in children. Pediatrics. 1981;67:828–32.
Grover M, Drossman DA. Psychotropic agents in function gastrointestinal disorders. Curr Opin Pharmacol. 2008;8(6):715–23.
Bursch B. Psychological/cognitive behavioral treatment of childhood functional abdominal pain and irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2008;47:706–7.
Whitfield KL, Shulman RJ. Treatment options for functional gastrointestinal disorders: from empiric to complementary approaches. Pediatr Ann. 2009;38(5):288–94.
Yin J, Abell TD, McCallum RW, et al. Gastric neuromodulation with Enterra system for nausea and vomiting in patients with gastroparesis. Neuromodulation. 2012;15(3):224–31.
Greenway F, Zheng J. Electrical stimulation as treatment for obesity and diabetes. J Diabetes Sci Technol. 2007;1(2):251–9.
Chen JDZ, Yin J, Wei W. Electrical therapies for gastrointestinal motility disorders. Expert Rev Gastroenterol Hepatol. 2017;11(4):407–18.
Apovian CM, Shah SN, Wolfe BM, et al. Two-year outcomes of vagal nerve blocking (vBloc) for the treatment of obesity in the ReCharge trial. Obes Surg. 2016;27:169–76.
Vu T, Lin F, Alshurafa N, et al. Wearable food intake monitoring technologies: a comprehensive review. Computers. 2017;6(1):4.
Amft O. A wearable ear pad sensor for chewing monitoring. Proceedings of the IEEE Sensors 2010 Conference; 2010 Nov 1-4; Waikoloa, USA IEEE Xplore. 2010. https://doi.org/10.1109/icsens.2010.5690449
Dong Y, Hoover A, Scisco J, et al. A new method for measuring meal intake in humans via automated wrist motion tracking. Appl Psychophysiol Biofeedback. 2012;37(3):205–15.
Farooq M, Fontana JM, Sazonov E. A novel approach for food intake detection using electroglottography. Physiol Meas. 2014;35(5):739–51.
Farooq M, Sazonov E. Comparative testing of piezoelectric and printed strain sensors in characterization of chewing. In: Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2015 Aug 25–29; Milano, Italy. IEEE Xplore; 2015:7538–7541
Dong B, Biswas S. Wearable diet monitoring through breathing signal analysis. In: Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2013 Jul 3–7; Osaka, Japan. IEEE Xplore; 2013:1186–1189.
Schwartz MW, Woods SC, Porte D, et al. Central nervous system control of food intake. Nature. 2000;404(6778):661–71.
Messina G, De Luca V, Viggiano A, et al. Autonomic nervous system in the control of energy balance and body weight: personal contributions. Neurol Res Int. 2013;2013:639280.
Sakaguchi T, Arase K, Fisler JS, et al. Effect of starvation and food intake on sympathetic activity. Am J Phys. 1988;255(2):R284–8.
Lu CL, Zou X, Orr WC, et al. Postprandial changes of sympathovagal balance measured by heart rate variability. Dig Dis Sci. 1999;44(4):857–61.
Stuckey MI, Tulppo MP, Kiviniemi AM, et al. Heart rate variability and the metabolic syndrome: a systematic review of the literature. Diabetes Metab Res Rev. 2014;30(8):784–93.
Blum A. Neural networks in C++: an object-oriented framework for building connectionist systems: Wiley; 1992.
Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng. 1985;32(3):230–6.
Welch P. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust. 1967;15(2):70–3.
Rangayyan BRM. Biomedical signal analysis: a case-study approach: IEEE Press Wiley-Interscience; 2002.
Heaton J. The Number of hidden layers [Internet]. Heaton Research; 2017 [cited 2018 Aug 1]. Available from: https://www.heatonresearch.com/2017/06/01/hidden-layers.html.
Hegenbart S, Uhl A, Vécsei A. Systematic assessment of performance prediction techniques in medical image classification: a case study on celiac disease. Inf Process Med Imaging. 2011;22:498–509.
Aberle J, Busch P, Veigel J, et al. Duodenal electric stimulation: results of a first-in-man study. Obes Surg. 2016;26:369–75.
Khawaled R, Blumen G, Fabricant G, et al. Intestinal electrical stimulation decreases postprandial blood glucose levels in rats. Surg Obes Relat Dis. 2009;5:692–7.
Ye F, Liu Y, Li S, et al. Hypoglycemic effects of intestinal electrical stimulation by enhancing nutrient-stimulated secretion of GLP-1 in rats. Obes Surg. 2018;28:2829–35.
Li S, Chen JD. Pulse width-dependent effects of intestinal electrical stimulation for obesity: role of gastrointestinal motility and hormones. Obes Surg. 2017;27:70–7.
Liu J, Xiang Y, Qiao X, et al. Hypoglycemic effects of intraluminal intestinal electrical stimulation in healthy volunteers. Obes Surg. 2011;21:224–30.
Chen JD, Lin Z, McCallum RW. Noninvasive feature-based detection of delayed gastric emptying in humans using neural networks. IEEE Trans Biomed Eng. 2000;47(3):409–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Heremans, E.R.M., Chen, A.S., Wang, X. et al. Artificial Neural Network-Based Automatic Detection of Food Intake for Neuromodulation in Treating Obesity and Diabetes. OBES SURG 30, 2547–2557 (2020). https://doi.org/10.1007/s11695-020-04511-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-020-04511-6