Abstract
Background
The hindgut theory hypothesizes a key role of differential hindgut stimulation following metabolic procedures in ameliorating diabetes mellitus. We used two strategies to remove the hindgut from intestinal continuity in order to analyze its impact on diabetes mellitus.
Methods
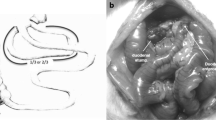
Loop duodeno-jejunostomy (DJOS) with exclusion of one-third of total intestinal length was performed in 3 groups of 9-week-old Zucker diabetic fatty rats. In group 1, no further alteration of the intestinal tract was made. Group 2 received additional ileal exclusion (IE). Group 3 underwent additional resection of 50% of the ileum with side-to-side ileocecal anastomosis (IR). One, 2, and 4 months after surgery, fasting blood glucose levels, oral glucose tolerance tests (OGTT), and glucose-stimulated hormone analyses were conducted, and bile acid blood levels were compared. Body weight was documented weekly.
Results
In relation to DJOS, glucose control was not impaired in IR or IE. On the contrary, only IR could maintain preOP glucose values until 4 months. There were no significant weight differences between the groups. Confirming effective ileal diversion, bile acid blood levels were significantly higher in the DJOS group compared with both IR and IE (p = 0.0025 and p = 0.0047). Operative interventions had no impact on GLP-1 levels at any time point (ANOVA p > 0.05 for all). Insulin secretion was preserved in all groups.
Conclusion
This data supports the hypothesis that the mechanisms driving amelioration of diabetes mellitus are complex and cannot be reduced to the ileum.






Similar content being viewed by others
References
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes — 5-year outcomes. N Engl J Med. 2017;376:641–51.
Mingrone G, Panunzi S, De Geaetano A, Guidone C, Ianconelli A, Nanni G. Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial - the lancet. Lancet 2015;386:964–973.
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–52.
Cummings DE, Rubino F. Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia. 2017;61:257–64.
Patriti A, Facchiano E, Sanna A, et al. The enteroinsular axis and the recovery from type 2 diabetes after bariatric surgery. Obes Surg. 2004;14:840–8.
Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Obes Surg. 2017;27:2–21.
Reimann F. Molecular mechanisms underlying nutrient detection by incretin-secreting cells. Int Dairy J. 2010;20:236–42.
Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–42.
Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554–9.
Amouyal C, Andreelli F. Increasing GLP-1 circulating levels by bariatric surgery or by GLP-1 receptor agonists therapy: why are the clinical consequences so different? J Diabetes Res. 2016;2016:5908656.
Imoto H, Shibata C, Ikezawa F, et al. Effects of duodeno-jejunal bypass on glucose metabolism in obese rats with type 2 diabetes. Surg Today. 2014;44:340–8.
Carmody JS, Muñoz R, Yin H, et al. Peripheral, but not central, GLP-1 receptor signaling is required for improvement in glucose tolerance after Roux-en-Y gastric bypass in mice. Am J Physiol Endocrinol Metab. 2016;310:E855–61.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
Hansen L, Lampert S, Hitoshi M, et al. Neural regulation of glucagon-like peptide-1 secretion in pigs. Am J Physiol Endocrinol Metab. 2004;287:E939–47.
Rocca A, Brubaker P. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1994;140:1687–94.
Laessle C, Michelmichel S, Marjanovic G, et al. Common channel length in bypass surgery does not impact T2DM in diabetic Zucker rats. Obes Surg. 2017;27:2090–8.
Laessle C, Nenova G, Marjanovic G, et al. Duodenal exclusion but not sleeve gastrectomy preserves insulin secretion, making it the more effective metabolic procedure. Obes Surg. 2018;28:1408–16.
Marjanovic G, Holzner P, Kulemann B, et al. Pitfalls and technical aspects during the research of intestinal anastomotic healing in rats. Eur Surg Res. 2010;45:314–20.
Shimabukuro M, Zhou Y-T, Levi M, et al. Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–502.
Grueneberger JM, Karcz-Socha I, Sawczyn T, et al. Systematic ileal transposition in Zucker rats shows advantage for long segment distal transposition. Surgery. 2014;155:165–72.
Dolo PR, Li C, Zhu X, et al. The effect of distal-ileal exclusion after Roux-en-Y gastric bypass on glucose tolerance and GLP-1 response in type-2 diabetes Sprague-Dawley rat model. Surg Obes Relat Dis. 2018;14:1552–60.
Buchwald H, Menchaca HJ, Michalek VN, et al. Ileal effect on blood glucose, HbA1c, and GLP-1 in Goto-Kakizaki rats. Obes Surg. 2014;24:1954–60.
Jurowich CF, Otto C, Rikkala PR, et al. Ileal interposition in rats with experimental type 2 like diabetes improves glycemic control independently of glucose absorption. J Diabetes Res. 2015;2015:490365.
Peiris M, Aktar R, Raynel S, et al. Effects of obesity and gastric bypass surgery on nutrient sensors, endocrine cells, and mucosal innervation of the mouse colon. Nutrients. 2018;10: https://doi.org/10.3390/nu10101529.
Liu Y, Zhou Y, Wang Y, et al. Roux-en-Y gastric bypass-induced improvement of glucose tolerance and insulin resistance in type 2 diabetic rats are mediated by glucagon-like peptide-1. Obes Surg. 2011;21:1424–31.
Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62:3044–52.
Flynn CR, Albaugh VL, Cai S, et al. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015;6:7715. https://doi.org/10.1038/ncomms8715.
Prawitt J, Caron S, Staels B. Glucose-lowering effects of intestinal bile acid sequestration through enhancement of splanchnic glucose utilization. Trends Endocrinol Metab. 2014;25:235–44.
Cummings BP, Bettaieb A, Graham JL, et al. Bile-acid-mediated decrease in endoplasmic reticulum stress: a potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis Model Mech. 2013;6:443–56.
Bhutta HY, Rajpal N, White W, et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLoS One. 2015;10: https://doi.org/10.1371/journal.pone.0122273.
Zhou Z, Kong F, Feng S, et al. Roux-en-Y gastric bypass surgery suppresses hepatic gluconeogenesis and increases intestinal gluconeogenesis in a T2DM rat model. Obes Surg. 2016;26:2683–90.
Albaugh VL, Banan B, Ajouz H, et al. Bile acids and bariatric surgery. Mol Asp Med. 2017;56:75–89.
Acknowledgments
The authors thank Silke Hempel for the outstanding work in assistance with ELISA measurements. Moreover, we thank Anja Schmitt for her excellent work in the establishment of the GLP-1 immunostaining and Claudia Bravo and Monika Kolterjahn for their outstanding care of our animals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent Statement
Does not apply.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laessle, C., Jin, K., Seifert, G.J. et al. Putting the Hindgut Hypothesis to the Test in a Diabetic Zucker Rat Model. OBES SURG 29, 4000–4007 (2019). https://doi.org/10.1007/s11695-019-04079-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04079-w