Abstract
Background
This paper aimed to evaluate the influence of modified biliopancreatic diversion (BPD) on the levels of GLP-1 and GLP-2 and correlate them with satiety regulation.
Methods
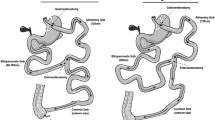
This is a pilot prospective cohort study that evaluated six mildly obese individuals with type 2 diabetes mellitus, which underwent modified BPD and were followed-up for 12 months. Levels of GLP-1 and GLP-2 after a standard meal tolerance test were determined and correlated with satiety scores obtained by means of a visual analogue scale (VAS).
Results
There were significant changes in BMI (33 ± 2.2 versus 26.3 ± 2.2 kg/m2; p < 0.001), HbA1c (7.9 ± 1.6 versus 5.8 ± 1.2%; p = 0.026), total cholesterol (172.3 ± 11.1 versus 134.7 ± 16.1 mg/dL; p < 0.001), LDL-c (103.3 ± 13 versus 64.6 ± 12.2 mg/dL; p < 0.001), and postprandial GLP-2 (972.7 ± 326.2 versus 1993.2 ± 1024.7; p = 0. 044). None of the scores obtained in the VAS significantly changed after surgery. After surgery, there were significant correlations of VAS scores and GLP-1 levels in question 01 (“how hungry do you feel?”; R = −0.928; p = .008) and GLP-2 levels in questions 02 (“how full do you feel?” R = 0.943; p = 0.005) and 04 (“how much do you think you can eat now? R = −0.829; p = 0.042).
Conclusions
Modified BPD does not lead to significant changes in satiety evaluated by the VAS; different aspects of satiety regulation are correlated with the postprandial levels of GLP-1 (hunger feeling) and GLP-2 (satiation feeling and desire to eat) 1 year after modified BPD, signaling a specific postoperative gut hormone-related modulation of appetite.



Similar content being viewed by others
References
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56.
Scopinaro N, Adami GF, Bruzzi P, et al. Prediction of diabetes remission at long term following biliopancreatic diversion. Obes Surg. 2017; doi:10.1007/s11695-017-2555-3. [Epub ahead of print]
Marceau P, Biron S, Hould FS, et al. Duodenal switch improved standard biliopancreatic diversion: a retrospective study. Surg Obes Relat Dis. 2009;5(1):43–7.
Marceau P, Biron S, Marceau S, et al. Long-term metabolic outcomes 5 to 20 years after biliopancreatic diversion. Obes Surg. 2015;25(9):1584–93.
Scopinaro N. Biliopancreatic diversion: mechanisms of action and long-term results. Obes Surg. 2006;16(6):683–9.
Scopinaro N. Thirty-five years of biliopancreatic diversion: notes on gastrointestinal physiology to complete the published information useful for a better understanding and clinical use of the operation. Obes Surg. 2012;22(3):427–32.
Strain GW, Torghabeh MH, Gagner M, et al. Nutrient status 9 years after biliopancreatic diversion with duodenal switch (BPD/DS): an observational study. Obes Surg. 2017; doi:10.1007/s11695-017-2560-6. [Epub ahead of print]
Topart P, Becouarn G, Delarue J. Weight loss and nutritional outcomes 10 years after biliopancreatic diversion with duodenal switch. Obes Surg. 2017; doi:10.1007/s11695-016-2537-x. [Epub ahead of print]
Sethi M, Chau E, Youn A, et al. Long-term outcomes after biliopancreatic diversion with and without duodenal switch: 2-, 5-, and 10-year data. Surg Obes Relat Dis. 2016;12(9):1697–705.
Cazzo E, Pareja JC, Chaim EA. Liver failure following biliopancreatic diversions: a narrative review. Sao Paulo Med J. 2017;135(1):66–70.
Geerts A, Darius T, Chapelle T, et al. The multicenter Belgian survey on liver transplantation for hepatocellular failure after bariatric surgery. Transplant Proc. 2010;42(10):4395–8.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg. 2017; doi:10.1007/s11695-017-2666-x. [Epub ahead of print]
Neff KJ, O'Shea D, le Roux CW. Glucagon like peptide-1 (GLP-1) dynamics following bariatric surgery: a signpost to a new frontier. Curr Diabetes Rev. 2013;9(2):93–101.
Tsoli M, Chronaiou A, Kehagias I, et al. Hormone changes and diabetes resolution after biliopancreatic diversion and laparoscopic sleeve gastrectomy: a comparative prospective study. Surg Obes Relat Dis. 2013;9(5):667–77.
Cazzo E, Pareja JC, Geloneze B, et al. Biliopancreatic diversion decreases postprandial apolipoprotein A-IV levels in mildly obese individuals with type 2 diabetes mellitus: a prospective study. Obes Surg. 2017;27(4):1008–12.
Borg CM, le Roux CW, Ghatei MA, et al. Biliopancreatic diversion in rats is associated with intestinal hypertrophy and with increased GLP-1, GLP-2 and PYY levels. Obes Surg. 2007;17(9):1193–8.
García-Unzueta MT, Fernández-Santiago R, Domínguez-Díez A, et al. Fasting plasma ghrelin levels increase progressively after biliopancreatic diversion: one-year follow-up. Obes Surg. 2005;15(2):187–90.
Garcia-Fuentes E, Garrido-Sanchez L, Garcia-Almeida JM, et al. Different effect of laparoscopic roux-en-Y gastric bypass and open biliopancreatic diversion of Scopinaro on serum PYY and ghrelin levels. Obes Surg. 2008;18(11):1424–9.
Dib N, Kiciak A, Pietrzak P, et al. Early-effect of bariatric surgery (Scopinaro method) on intestinal hormones and adipokines in insulin resistant Wistar rat. J Physiol Pharmacol. 2013;64(5):571–7.
Cazzo E, Pareja JC, Geloneze B, et al. Postprandial GLP-2 levels are increased after Biliopancreatic diversion in diabetic individuals with class I obesity: a prospective study. Obes Surg. 2017; doi:10.1007/s11695-017-2554-4. [Epub ahead of print]
Meek CL, Lewis HB, Reimann F, et al. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37.
Cazzo E, Pareja JC, Chaim EA, et al. GLP-1 and GLP-2 levels are correlated with satiety regulation after roux-en-Y gastric bypass: results of an exploratory prospective study. Obes Surg. 2017;27(3):703–8.
Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–37.
Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 2014;76:561–83.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists' comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract. 2013;19(3):536–57.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Flint A, Raben A, Blundell JE, et al. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48.
de Graaf C. The validity of appetite ratings. Appetite. 1993;21(2):156–60.
Pardina E, López-Tejero MD, Llamas R, et al. Ghrelin and apolipoprotein AIV levels show opposite trends to leptin levels during weight loss in morbidly obese patients. Obes Surg. 2009;19(10):1414–23.
Rowston W, McCluskey S, Gazet JC, et al. Eating behavior, physical symptoms and psychological factors associated with weight reduction following the Scopinaro operation as modified by Gazet. Obes Surg. 1992;2(4):355–60.
Adami GF, Gandolfo P, Cocchi FH, et al. Binge eating following biliopancreatic diversion for obesity. Appetite. 1995;25(2):177–88.
Kamiji MM, Troncon LE, Suen VM, et al. Gastrointestinal transit, appetite, and energy balance in gastrectomized patients. Am J Clin Nutr. 2009;89(1):231–9.
Jeon TY, Lee S, Kim HH, et al. Long-term changes in gut hormones, appetite and food intake 1 year after subtotal gastrectomy with normal body weight. Eur J Clin Nutr. 2010;64(8):826–31.
Kotidis EV, Koliakos G, Papavramidis TS, et al. The effect of biliopancreatic diversion with pylorus-preserving sleeve gastrectomy and duodenal switch on fasting serum ghrelin, leptin and adiponectin levels: is there a hormonal contribution to the weight-reducing effect of this procedure? Obes Surg. 2006 May;16(5):554–9.
Plourde CÉ, Grenier-Larouche T, Caron-Dorval D, et al. Biliopancreatic diversion with duodenal switch improves insulin sensitivity and secretion through caloric restriction. Obesity (Silver Spring). 2014;22(8):1838–46.
Gutzwiller JP, Drewe J, Göke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Phys. 1999;276(5 Pt 2):R1541–4.
Baggio LL, Huang Q, Brown TJ, et al. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53(9):2492–500.
ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia. 2015;58(12):2688–98.
Schmidt PT, Näslund E, Grybäck P, et al. Peripheral administration of GLP-2 to humans has no effect on gastric emptying or satiety. Regul Pept. 2003;116(1–3):21–5.
Sørensen LB, Flint A, Raben A, et al. No effect of physiological concentrations of glucagon-like peptide-2 on appetite and energy intake in normal weight subjects. Int J Obes Relat Metab Disord. 2003;27(4):450–6.
Scopinaro N, Adami GF, Marinari GM, et al. Biliopancreatic diversion. World J Surg. 1998 Sep;22(9):936–46.
Papadia FS, Elghadban H, Weiss A, et al. BPD and BPD-DS concerns and results. In: Huang C, editor. Advanced bariatric and metabolic surgery. Rijeka: Intech Open; 2012. p. 175–210.
Crea N, Pata G, Di Betta E, et al. Long-term results of biliopancreatic diversion with or without gastric preservation for morbid obesity. Obes Surg. 2011 Feb;21(2):139–45.
Cohen RV, Shikora S, Petry T, et al. The diabetes surgery summit II G7uidelines: a disease-based clinical recommendation. Obes Surg. 2016 Aug;26(8):1989–91.
Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016 Jun;39(6):861–77.
Scopinaro N, Adami GF, Papadia FS, et al. The effects of biliopancreatic diversion on type 2 diabetes mellitus in patients with mild obesity (BMI 30-35 kg/m2) and simple overweight (BMI 25-30 kg/m2): a prospective controlled study. Obes Surg. 2011;21(7):880–8.
Astiarraga B, Gastaldelli A, Muscelli E, et al. Biliopancreatic diversion in nonobese patients with type 2 diabetes: impact and mechanisms. J Clin Endocrinol Metab. 2013;98(7):2765–73.
Fried M, Ribaric G, Buchwald JN, et al. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI <35 kg/m2: an integrative review of early studies. Obes Surg. 2010;20(6):776–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Statement of Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP, protocol 2009/50430-6.
Rights and permissions
About this article
Cite this article
Cazzo, E., Pareja, J.C., Chaim, E.A. et al. Glucagon-Like Peptides 1 and 2 Are Involved in Satiety Modulation After Modified Biliopancreatic Diversion: Results of a Pilot Study. OBES SURG 28, 506–512 (2018). https://doi.org/10.1007/s11695-017-2875-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2875-3