Abstract
Background
This study aims to compare the post-prandial curves of glucose, insulin, GLP-1, and GLP-2 among individuals with Crohn’s disease (CD), obese individuals before and after bariatric surgery, and healthy controls.
Methods
This an exploratory cross-sectional study that involved five groups of patients (two groups of individuals with CD—active and inactive), bariatric patients (pre- and post-surgery, who were their own controls), and a distinct separated control group of healthy volunteers. C-reactive protein (CRP) levels and the post-prandial curves of glucose, insulin, GLP-1, and GLP-2 curves were assessed and compared.
Results
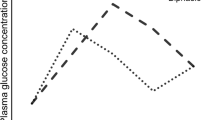
The pre-RYGB group presented significantly higher levels of CRP than the post-RYGB (p = 0.001) and the control group (p = 0.001). The inactive CD group presented a higher post-prandial GLP-1 area under the curve (AUC) than the pre-RYGB group (p = 0.009). The post-RYGB group presented significantly higher AUCs of GLP-2 than the pre-RYGB group (p < 0.0001), both inactive and active CD groups (p < 0.0001 in both situations), and the control group (p = 0.002). The pre-RYGB group presented a significantly higher AUC of glucose than the post-RYGB (p = 0.02) and both active and inactive CD groups (p = 0.019 and p = 0.046, respectively). The pre-RYGB group presented a significantly higher AUC of insulin than the control (p = 0.005) and both CD groups (p < 0.0001).
Conclusions
Obesity is associated with an inflammatory state comparable to the one observed in CD; inflammation may also be enrolled in the blockade of GLP-2. CD individuals present a more incretin-driven pattern of glucose metabolism, as a way to prevent hypoglycemia and compensate the carbohydrate malabsorption and GLP-2 blockade.


Similar content being viewed by others
References
World Health Organization. Global status report on noncommunicable diseases 2014. WHO: Geneva; 2014. p. 1–298.
Singh S, Dulai PS, Zarrinpar A, et al. Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017 Feb;14(2):110–21.
Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.e42. quiz e30
Nic Suibhne T, Raftery TC, McMahon O, et al. High prevalence of overweight and obesity in adults with Crohn’s disease: associations with disease and lifestyle factors. J Crohns Colitis. 2013;7(7):e241–8.
Bernstein GR, Pickett-Blakely O. De novo inflammatory bowel disease after bariatric surgery: a case series and literature review. Dig Dis Sci. 2016; https://doi.org/10.1007/s10620-016-4412-y.
Janczewska I, Nekzada Q, Kapraali M. Crohn’s disease after gastric bypass surgery. BMJ Case Rep. 2011; https://doi.org/10.1136/bcr.07.2010.3168.
Ahn LB, Huang CS, Forse RA, et al. Crohn’s disease after gastric bypass surgery for morbid obesity: is there an association? Inflamm Bowel Dis. 2005;11(6):622–4.
Kotze PG, Bremer-Nones R, Kotze LM. Is there any relation between gastric bypass for morbid obesity and the development of Crohn’s disease? J Crohns Colitis. 2014;8(7):712–3.
Shoar S, Shahabuddin Hoseini S, Naderan M, et al. Bariatric surgery in morbidly obese patients with inflammatory bowel disease: a systematic review. Surg Obes Relat Dis. 2017;13(4):652–9.
Harper JW, Zisman TL. Interaction of obesity and inflammatory bowel disease. World J Gastroenterol. 2016;22(35):7868–81.
Bregenzer N, Hartmann A, Strauch U, et al. Increased insulin resistance and beta cell activity in patients with Crohn’s disease. Inflamm Bowel Dis. 2006;12(1):53–6.
Nagahori M, Hyun SB, Totsuka T, et al. Prevalence of metabolic syndrome is comparable between inflammatory bowel disease patients and the general population. J Gastroenterol. 2010;45(10):1008–13.
Karrasch T, Obermeier F, Straub RH. Systemic metabolic signaling in acute and chronic gastrointestinal inflammation of inflammatory bowel diseases. Horm Metab Res. 2014;46(6):445–51.
Meek CL, Lewis HB, Reimann F, et al. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37.
Lee J, Koehler J, Yusta B, et al. Enteroendocrine-derived glucagon-like peptide-2 controls intestinal amino acid transport. Mol Metab. 2017; https://doi.org/10.1016/j.molmet.2017.01.005.
Cazzo E, Pareja JC, Chaim EA. Weight loss surgery and the surrogate insulin resistance markers HOMA, TyG, and TG/HDL-c in relation to metabolic syndrome. In: Preedy VR, Rajendram R, Martin CR. Metabolism and pathophysiology of bariatric surgery: nutrition, procedures, outcomes and adverse effects. London: Academic Press; 2017. p. 325–31. https://doi.org/10.1016/B978-0-12-804011-9.00055-8.
Jacobsen SH, Olesen SC, Dirksen C, et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22(7):1084–96.
Cazzo E, Gestic MA, Utrini MP, et al. GLP-2: a poorly understood mediator enrolled in various bariatric/metabolic surgery-related pathophysiologic mechanisms. Arq Bras Cir Dig. 2016;29(4):272–5.
LeRoux CW, Borg C, Wallis K, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252(1):50–6.
Taqi E, Wallace LE, de Heuvel E, et al. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg. 2010;45(5):987–95.
Cazzo E, Pareja JC, Geloneze B, et al. Postprandial GLP-2 levels are increased after biliopancreatic diversion in diabetic individuals with class I obesity: a prospective study. Obes Surg. 2017; https://doi.org/10.1007/s11695-017-2554-4.
Suzuki S, Ramos EJ, Gonçalves CG, et al. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery. 2005;138(2):283–90.
Huda MS, Wilding JP, Pinkney JH. Gut peptides and the regulation of appetite. Obes Rev. 2006;7(2):163–82.
Vahl T, D'Alessio D. Enteroinsular signaling: perspectives on the role of the gastrointestinal hormones glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide in normal and abnormal glucose metabolism. Curr Opin Clin Nutr Metab Care. 2003;6(4):461–8.
Bendet N, Scapa E, Cohen O, et al. Enhanced glucose-dependent glucagon-like peptide-1 and insulin secretion in Crohn patients with terminal ileum disease is unrelated to disease activity or ileal resection. Scand J Gastroenterol. 2004;39(7):650–6.
Tsukahara T, Watanabe K, Watanabe T, et al. Tumor necrosis factor α decreases glucagon-like peptide-2 expression by up-regulating G-protein-coupled receptor 120 in Crohn disease. Am J Pathol. 2015;185:185–96.
Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–44.
Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–53.
National Institutes of Health. Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr 1992;55(2 Suppl):615S–619S.
Lee WJ, Huang MT, Wang W, et al. Effects of obesity surgery on the metabolic syndrome. Arch Surg. 2004;139(10):1088–92.
Dorman RB, Serrot FJ, Miller CJ, et al. Case-matched outcomes in bariatric surgery for treatment of type 2 diabetes in the morbidly obese patient. Ann Surg. 2012;255(2):287–93.
Mumme DE, Mathiason MA, Kallies KJ, et al. Effect of laparoscopic Roux-en-Y gastric bypass surgery on hemoglobin A1c levels in diabetic patients: a matched-cohort analysis. Surg Obes Relat Dis. 2009;5(1):4–10.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61.
Batsis JA, Romero-Corral A, Collazo-Clavell ML, et al. Effect of bariatric surgery on the metabolic syndrome: a population-based, long-term controlled study. Mayo Clin Proc. 2008;83(8):897–907.
Inabnet 3rd WB, Winegar DA, Sherif B, et al. Early outcomes of bariatric surgery in patients with metabolic syndrome: an analysis of the bariatric outcomes longitudinal database. J Am Coll Surg. 2012;214(4):550–6.
Cazzo E, Gestic MA, Utrini MP, et al. Influence of insulin resistance status on the development of gallstones following Roux-en-Y gastric bypass: a prospective cohort study. Obes Surg. 2016;26(4):769–75.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg. 2017; https://doi.org/10.1007/s11695-017-2666-x.
de Hollanda A, Jiménez A, Corcelles R, et al. Gastrointestinal hormones and weight loss response after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2014;10(5):814–9.
le Roux CW, Borg C, Wallis K, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252(1):50–6.
Romero F, Nicolau J, Flores L, et al. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-en-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26(8):2231–9.
Cazzo E, Pareja JC, Chaim EA, et al. GLP-1 and GLP-2 levels are correlated with satiety regulation after Roux-en-Y gastric bypass: results of an exploratory prospective study. Obes Surg. 2016; https://doi.org/10.1007/s11695-016-2345-3.
Tigas S, Tsatsoulis A. Endocrine and metabolic manifestations in inflammatory bowel disease. Ann Gastroenterol. 2012;25(1):37–44.
Gonçalves P, Magro F, Martel F. Metabolic inflammation in inflammatory bowel disease: crosstalk between adipose tissue and bowel. Inflamm Bowel Dis. 2015;21(2):453–67.
Sellin JH, Hart R. Glucose malabsorption associated with rapid intestinal transit. Am J Gastroenterol. 1992;87(5):584–9.
Bjørneklett A, Fausa O, Midtvedt T. Bacterial overgrowth in jejunal and ileal disease. Scand J Gastroenterol. 1983;18(2):289–98.
Raithel M, Weidenhiller M, Hagel AF, et al. The malabsorption of commonly occurring mono and disaccharides: levels of investigation and differential diagnoses. Dtsch Arztebl Int. 2013;110(46):775–82.
Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol. 2012;590(5):1035–44.
Tamura A, Hayashi H, Imasato M, et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140(3):913–23.
Caradonna L, Amati L, Magrone T, et al. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6(3):205–14.
Herlitz-Cifuentes HS, Garces PC, Fernandez LI, et al. Effect of systemic inflammation on the function of insulin and glucose metabolism in rheumatoid arthritis. Curr Diabetes Rev. 2015;12(2):156–62.
Guerrero-Romero F, Simental-Mendía LE, Rodríguez-Morán M. Association of C-reactive protein levels with fasting and postload glucose levels according to glucose tolerance status. Arch Med Res. 2014;45(1):70–5.
Schmidt PT, Ljung T, Hartmann B, et al. Tissue levels and post-prandial secretion of the intestinal growth factor, glucagon-like peptide-2, in controls and inflammatory bowel disease: comparison with peptide YY. Eur J Gastroenterol Hepatol. 2005;17:207–12.
Sigalet DL, Kravarusic D, Butzner D, et al. A pilot study examining the relationship among Crohn disease activity, glucagon-like peptide-2 signalling and intestinal function in pediatric patients. Can J Gastroenterol. 2013;27:587–92.
Coy CSR, Calixto A, Vasques AC, et al. Evaluation of GLP-2 levels in Crohn’s disease. Inflamm Bowel Dis. 2016;22(Suppl 1):S58.
Divella R, De Luca R, Abbate I, et al. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer. 2016;7(15):2346–59.
Cooke AA, Connaughton RM, Lyons CL, et al. Fatty acids and chronic low grade inflammation associated with obesity and the metabolic syndrome. Eur J Pharmacol. 2016;785:207–14.
Tarantino G. Gut microbiome, obesity-related comorbidities, and low-grade chronic inflammation. J Clin Endocrinol Metab. 2014;99(7):2343–6.
van Avesaat M, Troost FJ, Ripken D, et al. Ileal brake activation: macronutrient-specific effects on eating behavior? Int J Obes. 2015;39(2):235–43.
Meloni AR, DeYoung MB, Lowe C, et al. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes Metab. 2013;15(1):15–27.
Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8(5):369–85.
Eriksson LS. Splanchnic exchange of glucose, amino acids and free fatty acids in patients with chronic inflammatory bowel disease. Gut. 1983;24(12):1161–8.
Lee YS, Shin S, Shigihara T, et al. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes. 2007;56(6):1671–9.
Zietek T, Daniel H. Intestinal nutrient sensing and blood glucose control. Curr Opin Clin Nutr Metab Care. 2015;18(4):381–8.
Magro D, Kotze P, Camargo M, et al. Serum levels of lipopolysaccharides and CD26 in patients with Crohn’s disease. Inflamm Bowel Dis. 2017;23(Suppl 1):S90.
Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Janowska J, et al. Serum concentrations of nitric oxide, tumor necrosis factor (TNF)-alpha and TNF soluble receptors in women with overweight and obesity. Metabolism. 2004;53(10):1268–73.
Cartier A, Lemieux I, Alméras N, et al. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-alpha in men. J Clin Endocrinol Metab. 2008;93(5):1931–8.
Gentile S, Guarino G, Bizzarro A, et al. Infliximab does not interfere with insulin secretion, insulin resistance and production of GAD and islet cell antibodies in patients with Crohn’s disease. Diabetes Obes Metab. 2002;4(4):276–7.
Rosenvinge A, Krogh-Madsen R, Baslund B, et al. Insulin resistance in patients with rheumatoid arthritis: effect of anti-TNFalpha therapy. Scand J Rheumatol. 2007;36(2):91–6.
Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9(1):25–53.
Pina T, Armesto S, Lopez-Mejias R, et al. Anti-TNF-α therapy improves insulin sensitivity in non-diabetic patients with psoriasis: a 6-month prospective study. J Eur Acad Dermatol Venereol. 2015;29(7):1325–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Paulo Gustavo Kotze is a speaker and consultant for Abbvie, Ferring, Janssen, and Takeda.
Other authors declare that they have no conflict of interest.
Statement of Informed Consent
Informed consent was obtained from all individual participants included in the study.
Statement of Human and Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Funding
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), protocols 2009/50430-6 and 2014/06164-8.
Rights and permissions
About this article
Cite this article
Magro, D.O., Cazzo, E., Kotze, P.G. et al. Glucose Metabolism Parameters and Post-Prandial GLP-1 and GLP-2 Release Largely Vary in Several Distinct Situations: a Controlled Comparison Among Individuals with Crohn’s Disease and Individuals with Obesity Before and After Bariatric Surgery. OBES SURG 28, 378–388 (2018). https://doi.org/10.1007/s11695-017-2851-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2851-y