Abstract
Purpose
The purpose of the study was to study the impact of the two most common bariatric surgery techniques on paracetamol pharmacokinetics (a marker of gastric emptying) and caffeine metabolism (a marker of liver function).
Materials and Methods
In the present prospective study, we studied 24 morbid obese patients before, at 4 weeks, and 6 months after having undergone sleeve gastrectomy (n = 10) or Roux-en-Y gastric bypass (n = 14). For comparative purposes, 28 healthy controls (14 normal weights and 14 overweights) were also included in the study.
Results
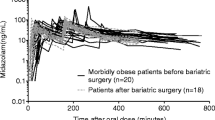
Paracetamol pharmacokinetics was altered in the obese participants leading to lower bioavailability. Bariatric surgery resulted in faster absorption and normalized pharmacokinetic parameters, prompting an increase in paracetamol bioavailability. No differences were found between surgical procedures. In the case of caffeine, the ratio paraxanthine/caffeine did not differ between morbid obese and healthy individuals. This ratio remained unmodified after surgery, indicating that the liver function (assessed by cytochrome P450 1A2 activity) was unaffected by obesity or bariatric surgery.
Conclusions
Paracetamol pharmacokinetics and caffeine plasma levels are altered in severely obese patients. The two studied bariatric surgical techniques normalize paracetamol oral bioavailability without impairing the liver function (measured by cytochrome P450 1A2 activity).



Similar content being viewed by others
References
Stein J, Stier C, Raab H, et al. Review article: the nutritional and pharmacological consequences of obesity surgery. Aliment Pharmacol Ther. 2014;40(6):582–609.
Greenblatt HK, Greenblatt DJ. Altered drug disposition following bariatric surgery: a research challenge. Clin Pharmacokinet. 2015;54(6):573–9.
Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev. 2010;11(1):41–50.
Prescott LF. Kinetics and metabolism of paracetamol and phenacetin. Brit J Clin Pharmacol. 1980;10(S2):291S–8S.
Raffa RB, Pergolizzi JV, Taylor R, et al. Acetaminophen (paracetamol) oral absorption and clinical influences. Pain Practice. 2014;14(7):668–77.
Heading RC, Nimmo J, Prescott LF, et al. The dependence of paracetamol absorption on the rate of gastric emptying. Br J Pharmacol. 1973;47(2):415–21.
Bartholomé R, Salden B, Vrolijk MF, et al. Paracetamol as a post prandial marker for gastric emptying, a food-drug interaction on absorption. PLoS One. 2015;10(9):e0136618.
Renner E, Wietholtz H, Huguenin P, et al. Caffeine: a model compound for measuring liver function. Hepatology. 1984;4(1):38–46.
Carrillo JA, Christensen M, Ramos SI, et al. Evaluation of caffeine as an in vivo probe for CYP1A2 using measurements in plasma, saliva, and urine. Ther Drug Monit. 2000;22(4):409–17.
Doude van Troostwijk LJ, Koopmans RP, Guchelaar H-J. Two novel methods for the determination of CYP1A2 activity using the paraxanthine/caffeine ratio. Fundam Clin Pharmacol. 2003;17(3):355–62.
Hubbard V, Hall H. Gastrointestinal surgery for severe obesity. Obes Surg. 1991;1(3):257–65.
Benaiges D, Goday A, Pedro-Botet J, et al. Bariatric surgery: to whom and when? Minerva Endocrinol. 2015;40(2):119–28.
Vidal P, Ramón JM, Goday A, et al. Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy as a definitive surgical procedure for morbid obesity. Mid-Term Results Obes Surg. 2013;23(3):292–9.
Christensen M, Andersson K, Dalén P, et al. The karolinska cocktail for phenotyping of five human cytochrome P450 enzymes. Clin Pharmacol Ther. 2003;73(6):517–28.
Hu OY-P, Tang H-S, Lane H-Y, et al. Novel single-point plasma or saliva dextromethorphan method for determining CYP2D6 activity. J Pharmacol Exp Ther. 1998;285(3):955–60.
Carbó M, Segura J, De la Torre R, et al. Effect of quinolones on caffeine disposition. Clin Pharmacol Ther. 1989;45(3):234–40.
Farré M, Roset P, Pascual J, et al. Estudio de la biodisponibilidad comparativa de la asociación de paracetamol y codeína (Gelocatil Codeína) frente a un preparado de referencia. Dolor. 1999;14:163–71.
Abernethy DR, Divoll M, Greenblatt DJ, et al. Obesity, sex, and acetaminophen disposition. Clin Pharmacol Ther. 1982;31(6):783–90.
Cappelletti S, Daria P, Sani G, et al. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol. 2015;13(1):71–88.
De Baerdemaeker LE, Mortier EP, Struys MM. Pharmacokinetics in obese patients. Contin Educ Anaesth Crit Care Pain. 2004;4(5):152–5.
Portolés A, Puerro M, Terleira A, et al. A new high-absorption-rate paracetamol 500-mg formulation: a comparative bioavailability study in healthy volunteers. Curr Ther Res. 2003;64(7):401–11.
Endrenyi L, Tothfalusi L. Metrics for the evaluation of bioequivalence of modified-release formulations. AAPS J. 2012;14(4):813–9.
Abernethy DR, Todd EL, Schwartz JB. Caffeine disposition in obesity. Br J Clin Pharmacol. 1985;20(1):61–6.
Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and Health outcomes at three years after bariatric surgery among patients with severe obesity. JAMA. 2013;310(22):2416–25.
Kushner RF, Sorensen KW. Prevention of weight regain following bariatric surgery. Curr Obes Rep. 2015;4(2):198–206.
Acknowledgements
This work was supported by a grant from the Instituto de Salud Carlos III, Fondo de Investigaciones Sanitarias, FISS ETS PI08/90638, JR14/00008 to OC, JR15/00005 to CPM, and JR16/00020 to EP. This work was also partially supported by grants MTM2015-64465-C2-1-R of the Ministerio de Economía y Competividad (Spain) and 2014 SGR 464 from the Departament d’Economia i Coneixement de la Generalitat de Catalunya (Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Clinical Trial Registration
The study was approved by the local Research Ethical Committee (CEIC-Parc de Salut Mar, Barcelona, Spain) and Spanish Medicines Agency (EudraCT 2009-013156-72) and registered as a clinical trial in a public database (ClinicalTrials.gov NCT01086722).
Ethical Statement
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent Statement
Informed consent was obtained from all individual participants included in the study.
Electronic Supplementary Material
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Goday Arno, A., Farré, M., Rodríguez-Morató, J. et al. Pharmacokinetics in Morbid Obesity: Influence of Two Bariatric Surgery Techniques on Paracetamol and Caffeine Metabolism. OBES SURG 27, 3194–3201 (2017). https://doi.org/10.1007/s11695-017-2745-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2745-z