Abstract
Objectives
We sought to investigate the short-term effect of weight loss following restrictive bariatric surgery on plasma concentrations of apelin and asymmetric dimethylarginine (ADMA) in individuals with morbid obesity.
Subjects/Methods
Thirty-seven morbidly obese individuals underwent laparoscopic greater curvature plication (LGCP). Anthropometric indices and plasma concentrations high-sensitivity C-reactive protein (hsCRP), apelin, and ADMA were measured before and 6 weeks after LGCP.
Results
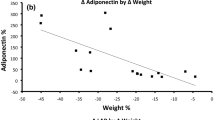
The percentage of total weight loss was 12.9 ± 4.4% 6 weeks after the operation. ADMA and apelin levels decreased significantly (p < 0.001 and 0.032, respectively) following LGCP. Significant decrements occurred in weight, body mass index, waist and hip circumference (p < 0.001), and waist-to-hip ratio (p = 0.013). The levels of triglycerides (p = 0.017), low-density lipoprotein cholesterol (p = 0.020), fasting plasma glucose (p = 0.033), fasting plasma insulin (p = 0.042), and the homeostasis model assessment index of insulin resistance (p = 0.034) also significantly decreased compared to the baseline measures. No significant change was observed in hsCRP levels and systolic and diastolic blood pressures. There was no significant correlation between changes in levels of apelin or ADMA and changes in anthropometric indices and other laboratory parameters.
Conclusions
Surgically induced weight loss rapidly decreases plasma levels of ADMA and apelin in morbidly obese patients. These changes do not seem correlated with changes in anthropometric and laboratory parameters associated with obesity.


Similar content being viewed by others
References
Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9(1):88.
Esteghamati A, Etemad K, Koohpayehzadeh J, et al. Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005–2011. Diabetes Res Clin Pract. 2014;103(2):319–27.
Ghoghaei M, Taghdiri F, Khajeh E, et al. Parathyroid hormone levels may predict nonalcoholic steatohepatitis in morbidly obese patients. Hepat Mon. 2015;15(7):e29697.
Van Gaal LF, Mertens IL, Christophe E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–80.
Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(03):347–55.
Vallance P, Leone A, Calver A, et al. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992;20:S60–S2.
Palomo I, Contreras A, Alarcon LM, et al. Elevated concentration of asymmetric dimethylarginine (ADMA) in individuals with metabolic syndrome. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society. 2011;24(4):224–8.
Surdacki A, Nowicki M, Sandmann J, et al. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J Cardiovasc Pharmacol. 1999;33(4):652–8.
Boger RH, Bode-Boger SM, Szuba A, et al. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98(18):1842–7.
Abbasi F, Asagmi T, Cooke JP, et al. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol. 2001;88(10):1201–3.
Vallance P, Leone A, Calver A, et al. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339(8793):572–5.
Krzyzanowska K, Mittermayer F, Kopp HP, et al. Weight loss reduces circulating asymmetrical dimethylarginine concentrations in morbidly obese women. J Clin Endocrinol Metab. 2004;89(12):6277–81.
Spoto B, Parlongo RM, Parlongo G, et al. The enzymatic machinery for ADMA synthesis and degradation is fully expressed in human adipocytes. Journal of nephrology. 2007;20(5):554.
Kimoto M, Whitley GSJ, Tsuji H, et al. Detection of NG, NGdimethylarginine dimethylaminohydrolase in human tissues using a monoclonal antibody. J Biochem. 1995;117(2):237–8.
Wilcken DE, Sim AS, Wang J, et al. Asymmetric dimethylarginine (ADMA) in vascular, renal and hepatic disease and the regulatory role of L-arginine on its metabolism. Mol Genet Metab. 2007;91(4):309–17.
Lu TM, Ding YA, Leu HB, et al. Effect of rosuvastatin on plasma levels of asymmetric dimethylarginine in patients with hypercholesterolemia. Am J Cardiol. 2004;94(2):157–61.
Mah E, Bruno RS. Postprandial hyperglycemia on vascular endothelial function: mechanisms and consequences. Nutr Res. 2012;32(10):727–40.
Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–6.
Masri B, Knibiehler B, Audigier Y. Apelin signalling: a promising pathway from cloning to pharmacology. Cell Signal. 2005;17(4):415–26.
Boucher J, Masri B, Daviaud D, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146(4):1764–71.
O’Carroll AM, Lolait SJ, Harris LE, et al. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol. 2013;219(1):R13–35.
Castan-Laurell I, Dray C, Attane C, et al. Apelin, diabetes, and obesity. Endocrine. 2011;40(1):1–9.
Castan-Laurell I, Boucher J, Dray C, et al. Apelin, a novel adipokine over-produced in obesity: friend or foe? Mol Cell Endocrinol. 2005;245(1–2):7–9.
Bertrand C, Valet P, Castan-Laurell I. Apelin and energy metabolism. Front Physiol. 2015;6:115.
Dray C, Knauf C, Daviaud D, et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008;8(5):437–45.
Attane C, Foussal C, Le Gonidec S, et al. Apelin treatment increases complete fatty acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes. 2012;61(2):310–20.
Monteforte MJ, Turkelson CM. Bariatric surgery for morbid obesity. Obes Surg. 2000;10(5):391–401.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Ghoghaei M, Khajeh E, Taghdiri F, et al. Effects of laparoscopic Roux-en-Y gastric bypass on anthropometric characteristics, hypertension, type 2 diabetes mellitus and metabolic syndrome: an Iranian experience. Galen Medical Journal. 2014;3(3):167–75.
Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg. 2009;19(12):1605–11.
Yermilov I, McGory ML, Shekelle PW, et al. Appropriateness criteria for bariatric surgery: beyond the NIH guidelines. Obesity. 2009;17(8):1521–7.
Talebpour M, Amoli BS. Laparoscopic total gastric vertical plication in morbid obesity. Journal of Laparoendoscopic & Advanced Surgical Techniques. 2007;17(6):793–8.
Talebpour M, SMK M, Talebpour A, et al. Twelve year experience of laparoscopic gastric plication in morbid obesity: development of the technique and patient outcomes. Ann Surg Innov Res. 2012;6(1):1.
Deitel M, Greenstein RJ. Recommendations for reporting weight loss. Obes Surg. 2003;13(2):159–60.
Schulze F, Wesemann R, Schwedhelm E, et al. Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clinical Chemical Laboratory Medicine. 2004;42(12):1377–83.
Konukoglu D, Uzun H, Firtina S, et al. Plasma adhesion and inflammation markers: asymmetrical dimethyl-L-arginine and secretory phospholipase A2 concentrations before and after laparoscopic gastric banding in morbidly obese patients. Obes Surg. 2007;17(5):672–8.
Krist J, Wieder K, Klöting N, et al. Effects of weight loss and exercise on apelin serum concentrations and adipose tissue expression in human obesity. Obesity facts. 2013;6(1):57–69.
Heinonen MV, Purhonen AK, Miettinen P, et al. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept. 2005;130(1–2):7–13.
Heinonen MV, Laaksonen DE, Karhu T, et al. Effect of diet-induced weight loss on plasma apelin and cytokine levels in individuals with the metabolic syndrome. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2009;19(9):626–33.
Caron-Cantin SM, Martin J, Bastien M, et al. Acute and chronic effects of biliopancreatic diversion with duodenal switch surgery on plasma visfatin and apelin levels in patients with severe obesity. Obes Surg. 2013;23(11):1806–14.
Soriguer F, Garrido-Sanchez L, Garcia-Serrano S, et al. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg. 2009;19(11):1574–80.
Zerrweck C, Rodriguez JG, Aramburo E, Vizcarra R, Rodriguez JL, Solorzano A, et al. Revisional surgery following laparoscopic gastric plication. Obes Surg 2016.
Spoto B, Parlongo RM, Parlongo G, et al. The enzymatic machinery for ADMA synthesis and degradation is fully expressed in human adipocytes. J Nephrol. 2007;20(5):554–9.
Wozniak SE, Gee LL, Wachtel MS, et al. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci. 2009;54(9):1847–56.
Castan-Laurell I, Vitkova M, Daviaud D, et al. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur J Endocrinol. 2008;158(6):905–10.
Zoccali C, Benedetto FA, Maas R, et al. Asymmetric dimethylarginine, C-reactive protein, and carotid intima-media thickness in end-stage renal disease. J Am Soc Nephrol. 2002;13(2):490–6.
Kadoglou NP, Lampropoulos S, Kapelouzou A, et al. Serum levels of apelin and ghrelin in patients with acute coronary syndromes and established coronary artery disease—KOZANI STUDY. Translational research : the journal of laboratory and clinical medicine. 2010;155(5):238–46.
El-Shehaby AM, El-Khatib MM, Battah AA, et al. Apelin: a potential link between inflammation and cardiovascular disease in end stage renal disease patients. Scand J Clin Lab Invest. 2010;70(6):421–7.
Mittermayer F, Mayer B, Meyer A, et al. Circulating concentrations of asymmetrical dimethyl-L-arginine are increased in women with previous gestational diabetes. Diabetologia. 2002;45(10):1372–8.
Fard A, Tuck CH, Donis JA, et al. Acute elevations of plasma asymmetric dimethylarginine and impaired endothelial function in response to a high-fat meal in patients with type 2 diabetes. Arterioscler Thromb Vasc Biol. 2000;20(9):2039–44.
Stühlinger MC, Abbasi F, Chu JW, et al. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA. 2002;287(11):1420–6.
Päivä H, Laakso J, Lehtimäki T, et al. Effect of high-dose statin treatment on plasma concentrations of endogenous nitric oxide synthase inhibitors. J Cardiovasc Pharmacol. 2003;41(2):219–22.
Eid HM, Eritsland J, Larsen J, et al. Increased levels of asymmetric dimethylarginine in populations at risk for atherosclerotic disease. Effects of pravastatin Atherosclerosis. 2003;166(2):279–84.
Laakso J, Ruokonen I, Rantalaiho V, et al. Plasma concentrations of asymmetric-dimethyl-arginine in type 2 diabetes associate with glycemic control and glomerular filtration rate but not with risk factors of vasculopathy. Metabolism. 2003;52(3):303–7.
Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63.
Päivä H, Lehtimäki T, Laakso J, et al. Dietary composition as a determinant of plasma asymmetric dimethylarginine in subjects with mild hypercholesterolemia. Metabolism. 2004;53(8):1072–5.
Acknowledgements
This study was funded by the Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences (Grant Number: 20476).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All study protocols were conducted in accordance with the principles of the Declaration of Helsinki 1972 and its subsequent revisions and were approved by the ethics committee of the Tehran University of Medical Sciences.
Informed Consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Elias Khajeh and Nekoo Panahi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Khajeh, E., Panahi, N., Golpaie, A. et al. Plasma Apelin and Asymmetric Dimethylarginine (ADMA) Levels Shortly After Laparoscopic Greater Curvature Plication. OBES SURG 27, 1596–1603 (2017). https://doi.org/10.1007/s11695-016-2509-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2509-1