Abstract
Background
Iron deficiency anemia (IDA) is a common finding in patients after bariatric surgery. The cause is multifactorial including reduced oral iron intake and malabsorption. While many patients can be managed with oral supplements, parenteral iron may be needed to restore and maintain iron stores.
Methods
Subjects who had previous bariatric surgery and had participated in phase 3 industry-sponsored clinical trials designed to assess the safety and/or efficacy of intravenous (IV) ferric carboxymaltose (FCM) were retrospectively selected from the databases of each of these studies. Demographic data, efficacy measures [hemoglobin, ferritin, and transferrin saturation (TSAT)], and adverse events were compared between FCM and other agents utilized as comparators in the trials.
Results
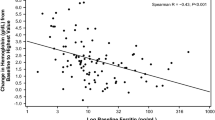
Two hundred eighty-one subjects from the intention to treat (ITT) population were included (mean age 49 years, BMI 33 kg/m2, including 253 females). FCM had similar or improved efficacy (p < 0.05) in terms of increasing hemoglobin, ferritin, and TSAT values when compared to other iron products used as standard of care for IDA. The incidence of adverse events in the FCM patients (n = 123) versus patients receiving any IV iron (n = 126) was 61 and 56.3 %, respectively. The adverse events were similar in both groups with the exception of a transient decrease in serum phosphate which was observed more frequently in the FCM group.
Conclusions
These data in post-bariatric surgery IDA patients suggest that FCM is a safe and effective alternative to existing iron products permitting higher and thus less frequent individual doses.
Similar content being viewed by others
References
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Longitudinal assessment of bariatric surgery. www2.NIDDK.nih.gov/publications/labs.htm. Accessed 20 Sep 2012.
Kaidar-Person O, Person B, Szomstein S, et al. Nutritional deficiencies in morbidly obese patients: a new form of malnutrition? Obes Surg. 2008;1028–34.
Skroubis G, Sakellaropoulos G, Pouggouras K, et al. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion. Obes Surg. 2002;12:551–8.
Dalcanale L, Oliveira CP, Faintuch J, et al. Long term nutritional outcome after gastric bypass. Obes Surg. 2010;20(2):181–7.
von Drygalski A, Andris DA, Nuttleman PR, et al. Anemia after bariatric surgery cannot be explained by iron deficiency alone: results of a large cohort study. Surg Obes Relat Dis. 2011;7:151–6.
Ruz M, Carrasco F, Rojas P, et al. Iron absorption and iron status are reduced after Roux-en-Y gastric bypass. Am J Clin Nutr. 2009;90(3):527–32.
Brolin RE, LaMarca LB, Kenler HA, et al. Malabsorptive gastric bypass in patients with super obesity. J Gastrointest Surg. 2002;6:195–203.
Brolin RE, Gorman JH, Gorman RC, et al. Are vitamin B12 and folate deficiency clinically important after Roux-en-Y gastric bypass? J Gastrointest Surg. 1998;2:436–42.
Rhode BM, Shustik C, Christou NV, et al. Iron absorption and therapy after gastric bypass. Obes Surg. 1999;9:17–21.
Vargas-Ruiz AG, Hernandez-Rivera G. Prevalence of iron, folate, and vitamin B12 deficiency anemia after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18:288–93.
Ganz T. Molecular control of iron transport. J Am Soc Nephrol. 2007;18:394–400.
Tussing-Humphreys LM, Nemeth E, Fantuzzi G, et al. Decreased serum hepcidin, inflammation, and improved functional iron status in obese pre-menopausal women six-months post-restrictive bariatric surgery. Obesity. 2010;18:2010–6.
Cheng HL, Bryant C, Cook R, et al. The relationship between obesity and hypoferraemia in adults: a systematic review. Obes Rev. 2012;13:150–61.
Mechanick JI, Kushner RF, Sugerman HJ, et al. AACE/TOS/ASMBS medical guidelines for clinical practice for the peri-operative nutritional, metabolic and nonsurgical support of the bariatric surgery patient. Endocr Pract. 2008;14:318–36.
Lyseng-Williamson KA, Keating GM. Ferric carboxymaltose: a review of its use in iron deficiency anemia. Drugs. 2009;69:739–56.
Barish CF, Koch T, Butcher A, et al. Safety and efficacy of intravenous ferric carboxymaltose (750 mg) in the treatment of iron deficiency anemia: two randomized controlled trials. Anemia. 2012;2012:172104.
Szczech LA, Bregman DB, Morris D, et al. Direct comparison of ferric carboxymaltose versus venofer in 2500 patients with iron deficiency anemia and impaired renal function (REPAIR-IDA). Kidney Week 2011 Abstract Supplement. J Am Soc Nephrol. 2011;22:5B. Available at http://www.asn-online.org/education/kidneyweek/archives/.
Szczech LA, Bregman DB, Morris D, et al. Comparison of high dose ferric carboxymaltose to oral or IV iron in subjects with iron deficiency anemia not suitable for oral iron. Kidney Week 2011 Abstract Supplement. J Am Soc Nephrol. 2011;22:435A. Available at http://www.asn-online.org/education/kidneyweek/archives/.
DeFilipp Z, Lister J, Gagne D, et al. Intravenous iron replacement for persistent iron deficiency anemia after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2013;9(1):129–32.
Van Wyck DB, Martens MG, Seid J, et al. Intravenous ferric carboxymaltose compared with oral iron in the treatment of post partum anemia: a randomized controlled trial. Obstet Gynecol. 2007;110(21):267–78.
Schouten BJ, Hunt PJ, Livesey JH, et al. FG23 elevation and hypophosphatemia after iron polymaltose: a prospective study. J Clin Endocrinol Metab. 2009;94(7):2332–7.
Conflict of Interest
Margaret Malone declares no conflict of interest; Charles Barish has received grant from Wake Research Associates and is a consultant for Luitpold Pharmaceuticals, Inc; Andy He is a post-doctoral fellow sponsored by St John’s University and Luitpold Pharmaceuticals, Inc.; and David Bregman is an employee of Luitpold Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Data were extracted from Luitpold Pharmaceuticals sponsored clinical trial database.
Rights and permissions
About this article
Cite this article
Malone, M., Barish, C., He, A. et al. Comparative Review of the Safety and Efficacy of Ferric Carboxymaltose Versus Standard Medical Care for the Treatment of Iron Deficiency Anemia in Bariatric and Gastric Surgery Patients. OBES SURG 23, 1413–1420 (2013). https://doi.org/10.1007/s11695-013-0939-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-013-0939-6