Abstract
Background
Gastric band operation and sleeve gastrectomy are increasingly popular bariatric surgeries for weight loss. The purpose of this study is to investigate the changes in plasma ghrelin levels and hypothalamic ghrelin receptor expression with weight loss achieved through these surgeries.
Methods
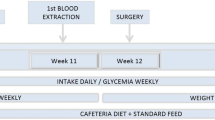
Twenty-four high fat diet-induced obese rats were used to investigate the effects of gastric band and sleeve operation on Body Mass Index, fat mass, plasma ghrelin levels, and hypothalamic growth hormone secretagogue receptor 1a (GHS-R 1a) protein expression in hypothalamus. In comparison, data of patients who received laparoscopic adjustable gastric banding (LAGB) and laparoscopic sleeve gastrectomy (LSG) in our hospital in 2005 were also summarized.
Results
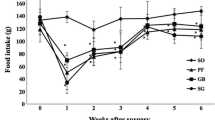
Body weights and fat mass decreased significantly in rats that received operation. Plasma ghrelin concentrations in the sleeve group were 0.4-fold of control rats and about 2-fold of control in the gastric band group. GHS-R1a protein expression in hypothalamus was 1.5-fold in the sleeve group compared with control group, while it was only 0.9-fold in the gastric band group. Clinical data showed that patients in the LSG group lost 60% excess body weights in 2 years follow-up. After operation, fasting plasma ghrelin concentrations in LAGB was significantly higher than the LSG group.
Conclusion
Both LAGB and LSG can decrease patients’ excess body weights and fat mass. Plasma ghrelin levels are down-regulated with LSG operation but up-regulated with LAGB operation. Hypothalamic GHS-R1a expression is elevated in sleeve gastrectomy.







Similar content being viewed by others
References
Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–7.
Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. Jama 2008;299:316–23.
Guijarro A, Kirchner H, Meguid MM. Catabolic effects of gastric bypass in a diet-induced obese rat model. Curr Opin Clin Nutr Metab Care 2006;9:423–35.
Chen CM. Overview of obesity in Mainland China. Obes Rev 2008;9(Suppl 1):14–21.
Suzuki S, Ramos EJ, Goncalves CG, et al. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery 2005;138:283–90.
Xu Y, Ramos EJ, Middleton F, et al. Gene expression profiles post Roux-en-Y gastric bypass. Surgery 2004;136:246–52.
Haslam DW, James WP. Obesity. Lancet 2005;366:1197–209.
Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics of obesity and diabetes in the United States. Jama 2001;286:1195–200.
Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science (New York, NY) 2005;307:1909–14.
Asakawa A, Inui A, Kaga T, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001;120:337–45.
Fukuda H, Mizuta Y, Isomoto H, et al. Ghrelin enhances gastric motility through direct stimulation of intrinsic neural pathways and capsaicin-sensitive afferent neurones in rats. Scand J Gastroenterol 2004;39:1209–14.
Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 2004;350:2549–57.
Kelley DE. Thermodynamics, liposuction, and metabolism. N Eng J Med 2004;350:2542–44.
Kirchner H, Guijarro A, Meguid MM. Is a model useful in exploring the catabolic mechanisms of weight loss after gastric bypass in humans. Curr Opin Clin Nutr Metab Care 2007;10:463–74.
Hill JO, Wyatt HR, Reed GW, et al. Obesity and the environment: where do we go from here. Science (New York, NY) 2003;299:853–5.
Steinbrook R. Surgery for severe obesity. N Eng J Med 2004;350:1075–9.
Pinkney JH, Sjostrom CD, Gale EA. Should surgeons treat diabetes in severely obese people. Lancet 2001;357:1357–9.
Cottam D, Qureshi FG, Mattar SG, et al. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc 2006;20:859–63.
Silecchia G, Boru C, Pecchia A, et al. Effectiveness of laparoscopic sleeve gastrectomy (first stage of biliopancreatic diversion with duodenal switch) on co-morbidities in super-obese high-risk patients. Obes Surg 2006;16:1138–44.
Deitel M, Crosby RD, Gagner M. The First International Consensus Summit for Sleeve Gastrectomy (SG), New York City, October 25–27, 2007. Obesity Surgery 2008.
Subramanian S, Han CY, Chiba T, et al. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 2008;28:685–91.
Monteiro MP, Ribeiro AH, Nunes AF, et al. Increase in ghrelin levels after weight loss in obese Zucker rats is prevented by gastric banding. Obes Surg 2007;17:1599–607.
Schmitz PG, O’Donnell MP, Kasiske BL, et al. Renal injury in obese Zucker rats: glomerular hemodynamic alterations and effects of enalapril. Am J Physiol 1992;263:F496–502.
Baltasar A, Deitel M, Greenstein RJ. Weight loss reporting. Obes Surg 2008;18:761–2.
Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ (Clinical research ed 1998;317:703–13).
Dixon JB, Pories WJ, O’Brien PE, et al. Surgery as an effective early intervention for diabesity: why the reluctance. Diabetes Care 2005;28:472–4.
Grassi G, Cattaneo BM, Seravalle G, et al. Obesity and the sympathetic nervous system. Blood Pressure 1996;1:43–6.
Peterli R, Wolnerhanssen BK, Peters T, et al. Prospective study of a two-stage operative concept in the treatment of morbid obesity: primary lap-band followed if needed by sleeve gastrectomy with duodenal switch. Obes Surg 2007;17:334–40.
Kotidis EV, Koliakos G, Papavramidis TS, et al. The effect of biliopancreatic diversion with pylorus-preserving sleeve gastrectomy and duodenal switch on fasting serum ghrelin, leptin and adiponectin levels: is there a hormonal contribution to the weight-reducing effect of this procedure. Obes Surg 2006;16:554–9.
Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med Soc Exp Biol Med (New York, NY) 1951;77:323–4.
Sahu A. Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology 2004;145:2613–20.
Frezza EE, Chiriva-Internati M, Wachtel MS. Analysis of the results of sleeve gastrectomy for morbid obesity and the role of ghrelin. Surg Today 2008;38:481–3.
Roa PE, Kaidar-Person O, Pinto D, et al. Laparoscopic sleeve gastrectomy as treatment for morbid obesity: technique and short-term outcome. Obes Surg 2006;16:1323–6.
Acknowledgements
The authors thank Professor Yun Yu for expert clinical assistance. The authors thank James Bosanquet of University of Missouri and Yan Zhang of Stanford University for revising this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Statement of Ethics
The study was approved by the China Medical University Institutional Ethics Committee.
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, J. Plasma Ghrelin Modulation in Gastric Band Operation and Sleeve Gastrectomy. OBES SURG 19, 357–362 (2009). https://doi.org/10.1007/s11695-008-9688-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-008-9688-3