Abstract
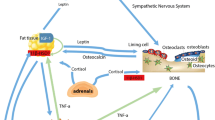
Body fat and lean mass are correlated with bone mineral density, with obesity apparently exerting protection against osteoporosis. The pathophysiological relevance of adipose tissue in bone integrity resides in the participation of adipokines in bone remodeling through effects on deposition and resorption. On the other hand, the skeleton has recently emerged as an endocrine organ with effects on body weight control and glucose homeostasis through the actions of bone-derived factors such as osteocalcin and osteopontin. The cross-talk between adipose tissue and the skeleton constitutes a homeostatic feedback system with adipokines and molecules secreted by osteoblasts and osteoclasts representing the links of an active bone–adipose axis. Given the impact of bariatric surgery on absorption and the adipokine secretory pattern, to focus on the changes taking place following surgical-induced weight loss on this dynamic system merits detailed consideration.

Similar content being viewed by others
References
Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801.
Rosen CJ, Bouxsein ML. Mehanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43.
Frühbeck G. Hunting for new pieces to the complex puzzle of obesity. Proc Nutr Soc. 2006;65:329–47.
Reid IR. Relationships between fat and bone. Osteoporos Int. 2008;19:595–606.
Zhao LJ, Liu YJ, Liu PY, et al. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92:1640–6.
Galusca B, Zouch M, Germain N, et al. Constitutional thinness: unusual human phenotype of low bone quality. J Clin Endocrinol Metab. 2008;93:110–7.
Hla MM, Davis JW, Ross PD, et al. A multicenter study of the influence of fat and lean mass on bone mineral content: evidence for differences in their relative influence at major fracture sites. Early Postmenopausal Intervention Cohort (EPIC) Study Group. Am J Clin Nutr. 1996;64:354–60.
Bélanger C, Luu-The V, Dupont P, et al. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm Metab Res. 2002;34:737–45.
Anandacoomarasamy A, Caterson I, Sambrook P, et al. The impact of obesity on the musculoskeletal system. Int J Obes. 2008;32:211–22.
Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, et al. The adipocyte: a model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;280:E827–47.
Gómez-Ambrosi J, Frühbeck G. Unlocking the molecular basis of obesity. Future Lipidol. 2007;2:577–81.
Oh KW, Lee WY, Rhee EJ, et al. The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin Endocrinol. 2005;63:131–8.
Misra M, Miller KK, Cord J, et al. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab. 2007;92:2046–52.
Peng XD, Xie H, Zhao Q, et al. Relationships between serum adiponectin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in Chinese men. Clin Chim Acta. 2008;387:31–5.
Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69.
Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20.
Steppan CM, Crawford DT, Chidsey-Frink KL, et al. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73–8.
Burguera B, Hofbauer LC, Thomas T, et al. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001;142:3546–53.
Hamrick MW, Della-Fera MA, Choi YH, et al. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005;20:994–1001.
Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207.
Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. 2006;4:341–8.
Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–17.
Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–20.
Fu L, Patel MS, Bradley A, et al. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–15.
Reid IR. Leptin deficiency-lessons in regional differences in the regulation of bone mass. Bone. 2004;34:369–71.
Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int. 2008;19:905–12.
Gordeladze JO, Reseland JE. A unified model for the action of leptin on bone turnover. J Cell Biochem. 2003;88:706–12.
Pasco JA, Henry MJ, Kotowicz MA, et al. Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab. 2001;86:1884–7.
Blain H, Vuillemin A, Guillemin F, et al. Serum leptin level is a predictor of bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2002;87:1030–5.
Thomas T, Burguera B, Melton LJ, 3rd et al. Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone. 2001;29:114–20.
Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74–80.
Berner HS, Lyngstadaas SP, Spahr A, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–9.
Shinoda Y, Yamaguchi M, Ogata N, et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006;99:196–208.
Luo XH, Guo LJ, Xie H, et al. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21:1648–56.
Oshima K, Nampei A, Matsuda M, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005;331:520–6.
Yamaguchi N, Kukita T, Li YJ, et al. Adiponectin inhibits induction of TNF-α/RANKL-stimulated NFATc1 via the AMPK signaling. FEBS Lett. 2008;582:451–6.
Luo XH, Guo LJ, Yuan LQ, et al. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005;309:99–109.
Lenchik L, Register TC, Hsu FC, et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33:646–51.
Jürimäe J, Jürimäe T. Adiponectin is a predictor of bone mineral density in middle-aged premenopausal women. Osteoporos Int. 2007;18:1253–9.
Richards JB, Valdes AM, Burling K, et al. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007;92:1517–23.
Tamura T, Yoneda M, Yamane K, et al. Serum leptin and adiponectin are positively associated with bone mineral density at the distal radius in patients with type 2 diabetes mellitus. Metabolism. 2007;56:623–8.
Gómez-Ambrosi J, Frühbeck G. Evidence for the involvement of resistin in inflammation and cardiovascular disease. Curr Diabetes Rev. 2005;1:227–34.
Thommesen L, Stunes AK, Monjo M, et al. Expression and regulation of resistin in osteoblasts and osteoclasts indicate a role in bone metabolism. J Cell Biochem. 2006;99:824–34.
Sethi JK, Vidal-Puig A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol Med. 2005;11:344–7.
Xie H, Tang SY, Luo XH, et al. Insulin-like effects of visfatin on human osteoblasts. Calcif Tissue Int. 2007;80:201–10.
Rodríguez A, Catalán V, Gómez-Ambrosi J, et al. Visceral and subcutaneous adiposity: are both potential therapeutic targets for tackling the metabolic syndrome? Curr Pharm Des. 2007;13:2169–75.
Wallenius V, Wallenius K, Ahren B, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–9.
Jilka RL, Hangoc G, Girasole G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91.
Franchimont N, Wertz S, Malaise M. Interleukin-6: an osteotropic factor influencing bone formation? Bone. 2005;37:601–6.
Papadopoulos NG, Georganas K, Skoutellas V, et al. Correlation of interleukin-6 serum levels with bone density in postmenopausal women. Clin Rheumatol. 1997;16:162–5.
Scheidt-Nave C, Bismar H, Leidig-Bruckner G, et al. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab. 2001;86:2032–42.
Khosla S, Peterson JM, Egan K, et al. Circulating cytokine levels in osteoporotic and normal women. J Clin Endocrinol Metab. 1994;79:707–11.
Bertolini DR, Nedwin GE, Bringman TS, et al. Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature. 1986;319:516–8.
Wennberg P, Nordstrom P, Lorentzon R, et al. TNF-α gene polymorphism and plasma TNF-α levels are related to lumbar spine bone area in healthy female Caucasian adolescents. Eur J Endocrinol. 2002;146:629–34.
Pfeilschifter J, Chenu C, Bird A, et al. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J Bone Miner Res. 1989;4:113–8.
Kobayashi K, Takahashi N, Jimi E, et al. Tumor necrosis factor a stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–86.
Catalán V, Gómez-Ambrosi J, Ramírez B, et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007;17:1464–74.
Martin TJ. A skeleton key to metabolism. Nat Med. 2007;13:1021–3.
Scatena M, Liaw L, Giachelli CM. Osteopontin. A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–9.
Reinholt FP, Hultenby K, Oldberg A, et al. Osteopontin—a possible anchor of osteoclasts to bone. Proc Natl Acad Sci USA. 1990;87:4473–5.
Gómez-Ambrosi J, Catalán V, Ramírez B, et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J Clin Endocrinol Metab. 2007;92:3719–27.
Nomiyama T, Perez-Tilve D, Ogawa D, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117:2877–88.
Kiefer FW, Zeyda M, Todoric J, et al. Osteopontin expression in human and murine obesity: extensive local upregulation in adipose tissue but minimal systemic alterations. Endocrinology. 2008;149:1350–7.
Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996;17:333–68.
Ferron M, Hinoi E, Karsenty G, et al. Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105:5266–70.
Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19.
Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–5.
Bucay N, Sarosi I, Dunstan CR, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8.
Holecki M, Zahorska-Markiewicz B, Janowska J, et al. The influence of weight loss on serum osteoprotegerin concentration in obese perimenopausal women. Obesity. 2007;15:1925–9.
Gannagé-Yared MH, Yaghi C, Habre B, et al. Osteoprotegerin in relation to body weight, lipid parameters insulin sensitivity, adipocytokines, and C-reactive protein in obese and non-obese young individuals: results from both cross-sectional and interventional study. Eur J Endocrinol. 2008;158:353–9.
Jono S, Ikari Y, Shioi A, et al. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106:1192–4.
Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001;86:631–7.
Kiechl S, Schett G, Wenning G, et al. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109:2175–80.
An JJ, Han DH, Kim DM, et al. Expression and regulation of osteoprotegerin in adipose tissue. Yonsei Med J. 2007;48:765–72.
Skopkova M, Penesova A, Sell H, et al. Protein array reveals differentially expressed proteins in subcutaneous adipose tissue in obesity. Obesity. 2007;15:2396–406.
Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–54.
Bradshaw AD, Graves DC, Motamed K, et al. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci USA. 2003;100:6045–50.
Delany AM, Amling M, Priemel M, et al. Osteopenia and decreased bone formation in osteonectin-deficient mice. J Clin Invest. 2000;105:915–23.
Tartare-Deckert S, Chavey C, Monthouel MN, et al. The matricellular protein SPARC/osteonectin as a newly identified factor up-regulated in obesity. J Biol Chem. 2001;276:22231–7.
Chavey C, Boucher J, Monthouel-Kartmann MN, et al. Regulation of secreted protein acidic and rich in cysteine during adipose conversion and adipose tissue hyperplasia. Obesity. 2006;14:1890–7.
Villareal DT, Fontana L, Weiss EP, et al. Bone mineral density response to caloric restriction-induced weight loss or exercise-induced weight loss: a randomized controlled trial. Arch Intern Med. 2006;166:2502–10.
Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59:151–84.
Pace DG, Blotner S, Guerciolini R. Short-term orlistat treatment does not affect mineral balance and bone turnover in obese men. J Nutr. 2001;131:1694–9.
Gotfredsen A, Westergren Hendel H, Andersen T. Influence of orlistat on bone turnover and body composition. Int J Obes Relat Metab Disord. 2001;25:1154–60.
Haney EM, Chan BK, Diem SJ, et al. Association of low bone mineral density with selective serotonin reuptake inhibitor use by older men. Arch Intern Med. 2007;167:1246–51.
Richards JB, Papaioannou A, Adachi JD, et al. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–94.
Pagotto U, Pasquali R. Fighting obesity and associated risk factors by antagonising cannabinoid type 1 receptors. Lancet. 2005;365:1363–4.
Tam J, Trembovler V, Di Marzo V, et al. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J. 2008;22:285–94.
Bab I, Zimmer A. Cannabinoid receptors and the regulation of bone mass. Br J Pharmacol. 2008;153:182–8.
Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18.
Murphy CE, Rodgers PT. Effects of thiazolidinediones on bone loss and fracture. Ann Pharmacother. 2007;41:2014–8.
Yaturu S, Bryant B, Jain SK. Thiazolidinedione treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care. 2007;30:1574–6.
Lin TH, Yang RS, Tang CH, et al. PPARγ inhibits osteogenesis via the down-regulation of the expression of COX-2 and iNOS in rats. Bone. 2007;41:562–74.
Wan Y, Chong LW, Evans RM. PPAR-γ regulates osteoclastogenesis in mice. Nat Med. 2007;13:1496–503.
Rosen CJ. Postmenopausal osteoporosis. N Engl J Med. 2005;353:595–603.
Coates PS, Fernstrom JD, Fernstrom MH, et al. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061–5.
Wucher H, Ciangura C, Poitou C, et al. Effects of weight loss on bone status after bariatric surgery: association between adipokines and bone markers. Obes Surg. 2008;18:58–65.
Kral JG, Näslund E. Surgical treatment of obesity. Nat Clin Pract Endocrinol Metab. 2007;3:574–83.
Saber AA, Elgamal MH, McLeod MK. Bariatric surgery: the past, present, and future. Obes Surg. 2008;18:121–8.
Guney E, Kisakol G, Ozgen G, et al. Effect of weight loss on bone metabolism: comparison of vertical banded gastroplasty and medical intervention. Obes Surg. 2003;13:383–8.
Olmos JM, Vazquez LA, Amado JA, et al. Mineral metabolism in obese patients following vertical banded gastroplasty. Obes Surg. 2008;18:197–203.
Strauss BJ, Marks SJ, Growcott JP, et al. Body composition changes following laparoscopic gastric banding for morbid obesity. Acta Diabetol. 2003;40 Suppl 1:S266–9.
von Mach MA, Stoeckli R, Bilz S, et al. Changes in bone mineral content after surgical treatment of morbid obesity. Metabolism. 2004;53:918–21.
Giusti V, Gasteyger C, Suter M, et al. Gastric banding induces negative bone remodelling in the absence of secondary hyperparathyroidism: potential role of serum C telopeptides for follow-up. Int J Obes. 2005;29:1429–35.
Goode LR, Brolin RE, Chowdhury HA, et al. Bone and gastric bypass surgery: effects of dietary calcium and vitamin D. Obes Res. 2004;12:40–7.
Ott MT, Fanti P, Malluche HH, et al. Biochemical evidence of metabolic bone disease in women following Roux-Y gastric bypass for morbid obesity. Obes Surg. 1992;2:341–8.
El-Kadre LJ, Rocha PRS, de Almeida Tinoco AC, et al. Calcium metabolism in pre- and postmenopausal morbidly obese women at baseline and after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2004;14:1062–6.
Riedt CS, Brolin RE, Sherrell RM, et al. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obes Res. 2006;14:1940–8.
Schweitzer DH. Mineral metabolism and bone disease after bariatric surgery and ways to optimize bone health. Obes Surg. 2007;17:1510–6.
Compher CW, Badellino KO, Boullata JI. Vitamin D and the bariatric surgical patient: a review. Obes Surg. 2008;18:220–4.
Duran de Campos C, Dalcanale L, Pajecki D, et al. Calcium intake and metabolic bone disease after eight years of Roux-en-Y gastric bypass. Obes Surg. 2008;18:386–90.
Johnson JM, Maher JW, Samuel I, et al. Effects of gastric bypass procedures on bone mineral density, calcium, parathyroid hormone, and vitamin D. J Gastrointest Surg. 2005;9:1106–10.
Goldner WS, Stoner JA, Thompson J, et al. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg. 2008;18:145–50.
Newbury L, Dolan K, Hatzifotis M, et al. Calcium and vitamin D depletion and elevated parathyroid hormone following biliopancreatic diversion. Obes Surg. 2003;13:893–5.
Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg. 2004;8:48–55.
Moreiro J, Ruiz O, Perez G, et al. Parathyroid hormone and bone marker levels in patients with morbid obesity before and after biliopancreatic diversion. Obes Surg. 2007;17:348–54.
Acknowledgments
The authors gratefully acknowledge the funding of their experimental work by grants from the Spanish Instituto de Salud Carlos III (FIS PI030381, FIS PI061458, and FIS PI06/90288) from the Ministerio de Sanidad y Consumo, as well as by grants 20/2005 and 3/2006 from the Department of Health of the Gobierno de Navarra, Spain and from the PIUNA Foundation. CIBER de Fisiopatología de la Obesidad y Nutrición (CIBEROBN) is an initiative of the Instituto de Salud Carlos III, Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors declare that they have nothing to disclose.
Rights and permissions
About this article
Cite this article
Gómez-Ambrosi, J., Rodríguez, A., Catalán, V. et al. The Bone-Adipose Axis in Obesity and Weight Loss. OBES SURG 18, 1134–1143 (2008). https://doi.org/10.1007/s11695-008-9548-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-008-9548-1