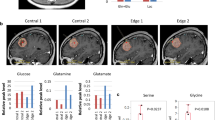
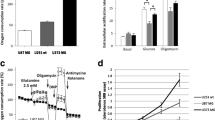
Abstract
Brain cancers demonstrate a complex metabolic behavior so as to adapt the external hypoxic environment and internal stress generated by reactive oxygen species. To survive in these stringent conditions, glioblastoma cells develop an antagonistic metabolic phenotype as compared to their predecessors, the astrocytes, thereby quenching the resources expected for nourishing the neurons. The complexity and cumulative effect of the large scale metabolic functioning of glioblastoma is mostly unexplored. In this study, we reconstruct a metabolic network comprising of pathways that are known to be deregulated in glioblastoma cells as compared to the astrocytes. The network, consisted of 147 genes encoding for enzymes performing 247 reactions distributed across five distinct model compartments, was then studied using constrained-based modeling approach by recreating the scenarios for astrocytes and glioblastoma, and validated with available experimental evidences. From our analysis, we predict that glycine requirement of the astrocytes are mostly fulfilled by the internal glycine–serine metabolism, whereas glioblastoma cells demand an external uptake of glycine to utilize it for glutathione production. Also, cystine and glucose were identified to be the major contributors to glioblastoma growth. We also proposed an extensive set of single and double lethal reaction knockouts, which were further perturbed to ascertain their role as probable chemotherapeutic targets. These simulation results suggested that, apart from targeting the reactions of central carbon metabolism, knockout of reactions belonging to the glycine–serine metabolism effectively reduce glioblastoma growth. The combinatorial targeting of glycine transporter with any other reaction belonging to glycine–serine metabolism proved lethal to glioblastoma growth.









Similar content being viewed by others
References
Anton K, Glod J (2014) An orchestrated response to tumor signals by macrophages and mesenchymal stem cells potentiates interleukin-6 secretion in glioblastoma. Cell Death Ther 1:2353–7817. doi:10.2478/cdth-2014-0001
Bairoch A (2000) The ENZYME database in 2000. Nucleic Acids Res 28:304–305
Banerji A (2013) An attempt to construct a (general) mathematical framework to model biological “context-dependence”. Syst Synth Biol 7:221–227
Boada J, Roig T, Perez X, Gamez A, Bartrons R, Cascante M, Bermúdez J (2000) Cells overexpressing fructose-2, 6-bisphosphatase showed enhanced pentose phosphate pathway flux and resistance to oxidative stress. FEBS Lett 480:261–264
Bouzier-Sore AK, Pellerin L (2013) Unraveling the complex metabolic nature of astrocytes. Front Cell Neurosci 7:179. doi:10.3389/fncel.2013.00179
Brekke E, Walls AB, Norfeldt L, Schousboe A, Waagepetersen HS, Sonnewald U (2012) Direct measurement of backflux between oxaloacetate and fumarate following pyruvate carboxylation. Glia 60:147–158. doi:10.1002/glia.21265
Burgess PK, Kulesa PM, Murray JD, Alvord EC Jr (1997) The interaction of growth rates and diffusion coefficients in a three-dimensional mathematical model of gliomas. J Neuropathol Exp Neurol 56:704–713
Chatterjee A, Mambo E, Sidransky D (2006) Mitochondrial DNA mutations in human cancer. Oncogene 25:4663–4674
Chinnaiyan P et al (2012) The metabolomic signature of malignant glioma reflects accelerated anabolic metabolism. Cancer Res 72:5878–5888
Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H (2005) Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci 25:7101–7110. doi:10.1523/JNEUROSCI.5258-04.2005
Consortium U (2014) Activities at the universal protein resource (UniProt). Nucleic Acids Res 42:D191–D198
Covert MW, Schilling CH, Palsson B (2001) Regulation of gene expression in flux balance models of metabolism. J Theor Biol 213:73–88
Deighton RF et al (2014) The proteomic response in glioblastoma in young patients. J Neurooncol 119:79–89. doi:10.1007/s11060-014-1474-6
Dringen R, Verleysdonk S, Hamprecht B, Willker W, Leibfritz D, Brand A (1998) Metabolism of glycine in primary astroglial cells: synthesis of creatine, serine, and glutathione. J Neurochem 70:835–840
Edgar R, Domrachev M, Lash AE (2002) Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30:207–210
García-Colunga J, Miledi R (1999) Modulation of nicotinic acetylcholine receptors by strychnine. Proc Natl Acad Sci 96:4113–4118
Griguer CE, Oliva CR, Gillespie GY (2005) Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neurooncol 74:123–133. doi:10.1007/s11060-004-6404-6
Guessous F et al (2013) Oncogenic effects of miR-10b in glioblastoma stem cells. J Neurooncol 112:153–163
Hashimoto K (2010) Glycine transport inhibitors for the treatment of schizophrenia. Open Med Chem 4:10–19
Hattingen E, Lanfermann H, Quick J, Franz K, Zanella FE, Pilatus U (2009) 1H MR spectroscopic imaging with short and long echo time to discriminate glycine in glial tumours. Magn Reson Mater Phys, Biol Med 22:33–41
Hertz L, Zielke HR (2004) Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci 27:735–743. doi:10.1016/j.tins.2004.10.008
Jellinger K (1977) Glioblastoma multiforme: morphology and biology. Acta Neurochir 42:5–32
Jeong H, Mason SP, Barabási A-L, Oltvai ZN (2001) Lethality and centrality in protein networks. Nature 411:41–42
Jursky F, Nelson N (1995) Localization of glycine neurotransmitter transporter (GLYT2) reveals correlation with the distribution of glycine receptor. J Neurochem 64:1026–1033
Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M (2014) Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res 42:D199–D205
Kleihues P, Ohgaki H (2000) Phenotype vs genotype in the evolution of astrocytic brain tumors. Toxicol Pathol 28:164–170
Lee JM, Gianchandani EP, Papin JA (2006) Flux balance analysis in the era of metabolomics. Brief Bioinform 7:140–150
Lehár J, Krueger AS, Avery W, Heilbut AM, Johansen LM, Price ER, Rickles RJ, Short GF 3rd, Staunton JE, Jin X, Lee MS, Zimmermann GR, Borisy AA (2009) Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat Biotechnol 27:659–666
Maity A, Pore N, Lee J, Solomon D, O’Rourke DM (2000) Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3′-kinase and distinct from that induced by hypoxia. Cancer Res 60:5879–5886
Mandonnet E, Pallud J, Clatz O, Taillandier L, Konukoglu E, Duffau H, Capelle L (2008) Computational modeling of the WHO grade II glioma dynamics: principles and applications to management paradigm. Neurosurg Rev 31:263–269
Mangia S, Simpson IA, Vannucci SJ, Carruthers A (2009) The in vivo neuron-to-astrocyte lactate shuttle in human brain: evidence from modeling of measured lactate levels during visual stimulation. J Neurochem 109(Suppl 1):55–62. doi:10.1111/j.1471-4159.2009.06003.x
Marrif H, Juurlink BH (1999) Astrocytes respond to hypoxia by increasing glycolytic capacity. J Neurosci Res 57:255–260
Nicklas WJ, Browning ET (1978) Amino acid metabolism in glial cells: homeostatic regulation of intra-and extracellular milieu by C-6 glioma cells. J Neurochem 30:955–963
Ogunrinu TA, Sontheimer H (2010) Hypoxia increases the dependence of glioma cells on glutathione. J Biol Chem 285:37716–37724. doi:10.1074/jbc.M110.161190
Oliveira-Ferrer L, Wellbrock J, Bartsch U, Penas EM, Hauschild J, Klokow M, Bokemeyer C, Fiedler W, Schuch G (2013) Combination therapy targeting integrins reduces glioblastoma tumor growth through antiangiogenic and direct antitumor activity and leads to activation of the pro-proliferative prolactin pathway. Mol Cancer 12:1–14
Oudard S et al (1996) High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer 74:839–845
Pelicano H, Martin D, Xu R, Huang P (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25:4633–4646
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci 91:10625–10629
Pistollato F et al (2010) Hypoxia and succinate antagonize 2-deoxyglucose effects on glioblastoma. Biochem Pharmacol 80:1517–1527. doi:10.1016/j.bcp.2010.08.003
Prabhu A, Sarcar B, Kahali S, Yuan Z, Johnson JJ, Adam KP, Kensicki E, Chinnaiyan P (2014) Cysteine catabolism: a novel metabolic pathway contributing to glioblastoma growth. Cancer Res 74:787–796
Raman K, Damaraju N, Joshi GK (2014) The organisational structure of protein networks: revisiting the centrality–lethality hypothesis. Syst Synth Biol 8:73–81
Resendis-Antonio O, Checa A, Encarnación S (2010) Modeling core metabolism in cancer cells: surveying the topology underlying the Warburg effect. PLoS ONE 5:e12383
Roux MJ, Supplisson S (2000) Neuronal and glial glycine transporters have different stoichiometries. Neuron 25:373–383
Sahm F et al (2013) The endogenous tryptophan metabolite and NAD + precursor quinolinic acid confers resistance of gliomas to oxidative stress. Cancer Res 73:3225–3234. doi:10.1158/0008-5472.CAN-12-3831
Schellenberger J et al (2011) Quantitative prediction of cellular metabolism with constraint-based models: the COBRA Toolbox v2. 0. Nat Protoc 6:1290–1307
Sonnewald U, Westergaard N, Jones P, Taylor A, Bachelard H, Schousboe A (1996) Metabolism of [U-13C5] glutamine in cultured astrocytes studied by NMR spectroscopy: first evidence of astrocytic pyruvate recycling. J Neurochem 67:2566–2572
Swanson KR, Bridge C, Murray J, Alvord EC (2003) Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J Neurol Sci 216:1–10
Tracqui P, Cruywagen G, Woodward D, Bartoo G, Murray J, Alvord E (1995) A mathematical model of glioma growth: the effect of chemotherapy on spatio-temporal growth. Cell Prolif 28:17–31
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1103. doi:10.1126/science.1160809
Wang D-S, Mangin J-M, Moonen G, Rigo J-M, Legendre P (2006) Mechanisms for picrotoxin block of α2 homomeric glycine receptors. J Biol Chem 281:3841–3855
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Wise DR et al (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA 105:18782–18787. doi:10.1073/pnas.0810199105
Wolf A, Agnihotri S, Guha A (2010) Targeting metabolic remodeling in glioblastoma multiforme. Oncotarget 1:552–577
Ye Z-C, Rothstein JD, Sontheimer H (1999) Compromised glutamate transport in human glioma cells: reduction–mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine–glutamate exchange. J Neurosci 19:10767–10777
Zafra F, Gimenez C (1986) Characterization of glycine uptake in plasma membrane vesicles isolated from cultured glioblastoma cells. Brain Res 397:108–116
Zhou Y et al (2011) Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. J Biol Chem 286:32843–32853. doi:10.1074/jbc.M111.260935
Acknowledgments
We thank Council of Scientific and Industrial Research, XII Five Year Plan Project “GENESIS” (BSC0121) and Department of Biotechnology, Government of India (Project Code: BT/PR13689/BID/07/363/2010) for providing financial support to perform this work. Abhishek Subramanian acknowledges the research fellowship provided by DBT-BINC fellowship program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhowmick, R., Subramanian, A. & Sarkar, R.R. Exploring the differences in metabolic behavior of astrocyte and glioblastoma: a flux balance analysis approach. Syst Synth Biol 9, 159–177 (2015). https://doi.org/10.1007/s11693-015-9183-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11693-015-9183-9