Abstract
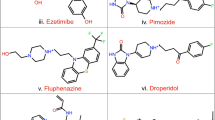
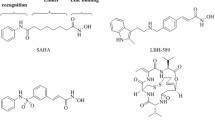
Sirtuins (class III histone deacetylase) are evolutionarily conserved NAD+-dependent enzymes that catalyze the deacetylation of acetyl-lysine residues of histones and other target proteins. Because of their associations in various pathophysiological conditions, the identification of small molecule modulators has been of significant interest. In the present study, virtual screening was carried out with NCI Diversity Set II using crystal structure of hSIRT2 (PDB ID: 1J8F) as a model for the docking procedure to find potential compounds, which were then subjected to experimental tests for their in vitro SIRT2 inhibitory activity. One of the 40 compounds tested, NSC671136 (IUPAC name: 6-Acetyl-4-oxo-1,3-diphenyl-2-thioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-5-yl 2,4-dichlorobenzoate) has structurally unique scaffold, showed strong inhibitory activity towards SIRT2 with IC50 of ~8.7 μM and to a lesser extent on SIRT1 activity. The reported compound is substantially potent compared to the published SIRT2 inhibitors and serves as an excellent base for future lead development.



Similar content being viewed by others
References
Cen Y (2009) Sirtuins inhibitors: the approach to affinity and selectivity. Biochim Biophys Acta 1804:1635–1644
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B et al (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305:390–392
Cosgrove MS, Bever K, Avalos JL, Muhammad S, Zhand X, Wolberger C (2006) The structural basis of sirtuin substrate affinity. Biochemistry 45:7511–7521
Cuperus G, Shafaatian R, Shore D (2000) Locus specificity determinants in the multifunctional yeast silencing protein Sir2. EMBO J 19:2641–2651
Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA (2003) Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol 23:3173–3185
Finnin MS, Donigian JR, Pavletich NP (2001) Structure of the histone deacetylase SIRT2. Nat Struct Biol 8:621–625
Heltweg B, Gatbonton T, Schuler AD, Posakony J, Li H, Goehle S, Kollipara R, DePinho RA, Gu Y, Simon JA, Bedalov A (2006) Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res 66:4368–4377
Huber K, Schemies J, Uciechowska U, Wagner JM, Rumpf T, Lewrick F, Seuss R, Sippl W, Jung M, Bracher F (2010) Novel 3-arylideneindolin-2-ones as inhibitors of NAD+-dependent histone deacetylases (sirtuins). J Med Chem 53:1383–1386
Huey R, Morris GM, Olson AJ, Goodsell DS (2007) A semi-empirical free energy force field with charge-based desolvation. J Comput Chem 28:1145–1152
Huhtiniemi T, Suuronen T, Valtteri MR, Wittekindt C, Lahtela-Kakkonen M, Jarho E, Walle′n EAA, Salminen A, Poso A, Leppänen J (2008) Oxadiazone-carbonylaminothioureas as SIRT1 and SIRT2 inhibitors. J Med Chem 51:4377–4380
Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403:795–800
Inoue T, Hiratsuka M, Osaki M, Oshimura M (2007) The molecular biology of mammalian SIRT proteins: SIRT2 in cell cycle regulation. Cell Cycle 6:1011–1108
Jin YH, Kim YJ, Kim DW, Baek KH, Kang BY, Yeo CY, Lee KY (2008) Sirt2 interacts with 14–3-3 beta/gamma and down-regulates the activity of p53. Biochem Biophys Res Commun 368:690–695
Johnstone RW (2002) Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov 1:287–299
Kiviranta PH, Leppanen J, Kyrylenko S, Salo HS, Lahtela-Kakkonen M, Tervo AJ, Wittekindt C, Suuronen T, Kuusisto E, Jarvinen T, Salminen A, Poso A, Wallen EA (2006) N,N′-Bisbenzylidenebenzene-1,4-diamines and N,N′-Bisbenzylidenenaphthalene-1,4-diamines as Sirtuin Type 2 (SIRT2) Inhibitors. J Med Chem 49:7907–7911
Kiviranta PH, Leppanen J, Rinne VM, Suuronen T, Kyrylenko O, Kyrylenko S, Kuusisto E, Tervo AJ, Jarvinen T, Salminen A, Poso A, Wallen EA (2007) N-(3-(4-Hydroxyphenyl)-propenoyl)-amino acid tryptamides as SIRT2 inhibitors. Bioorg Med Chem Lett 17:2448–2451
Lavu S, Boss O, Elliott PJ, Lambert PD (2008) Sirtuins—novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov 7:841–853
Marks PA, Richon VM, Miller T, Kelly WK (2004) Histone deacetylase inhibitors. Adv Cancer Res 91:137–168
McLaughlin F, La Thangue NB (2004) Histone deacetylase inhibitors open new doors in cancer therapy. Biochem Pharmacol 68:1139–1144
Milne JC, Denu JM (2008) The Sirtuin family: therapeutic targets to treat diseases of aging. Curr Opin Chem Biol 12:11–17
Min J, Landry J, Sternglanz R, Xu RM (2001) Crystal structure of a SIR2 homolog-NAD complex. Cell 105:269–279
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Algorithm and empirical binding free energy function. J Comput Chem 19:1639–1662
North BJ, Verdin E (2007) Interphase nucleocytoplasmic shuttling and localization of SIRT2 during mitosis. PLoS ONE 2:e784
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11:437–444
Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317:516–519
Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T et al (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429:771–776
Sanders BD, Jackson B, Marmorstein R (2010) Structural basis for sirtuin function: what we know and what we don’t, Biochim. Biophys Acta 1804:1604–1616
Tervo AJ, Kyrylenko S, Niskanen P, Salminen A, Leppanen J, Nyronen TH, Jarvinen T, Poso A (2004) An in silico approach to discovering novel inhibitors of human sirtuin type 2. J Med Chem 47:6292–6298
Tissenbaum HA, Guarente L (2001) Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410:227–230
Yang XJ, Seto E (2007) HATs and HDACs: from structure, function and regulation to novel strategies for therapy and prevention. Oncogene 26:5310–5318
Acknowledgments
We thank Drs. Javed Iqbal and Devyani Haldar for allowed us to utilize their biological facilities as well as for their constant support during our experimental work at Institute of Life Sciences (ILS, a not-for-profit organization), University of Hyderabad campus, Gachibowli, Hyderabad.
Author information
Authors and Affiliations
Corresponding author
Additional information
Padavattan Sivaraman and Suresh Mattegunta contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sivaraman, P., Mattegunta, S., Subbaraju, G.V. et al. Design of a novel nucleoside analog as potent inhibitor of the NAD+ dependent deacetylase, SIRT2. Syst Synth Biol 4, 257–263 (2010). https://doi.org/10.1007/s11693-011-9069-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11693-011-9069-4