Abstract
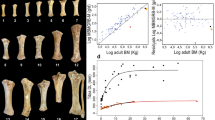
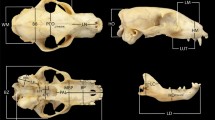
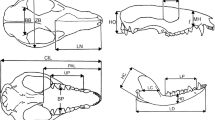
Members of the mammalian families Elephantidae and Hippopotamidae (extant and extinct elephants and hippos) include extinct dwarf species that display up to 98% decrease in body size compared to probable ancestral sources. In addition to differences in body mass, skulls of these species consistently display distinctive morphological changes, including major reduction of pneumatised areas in dwarf elephants and shortened muzzles in dwarf hippos. Here we build on previous studies of island dwarf species by conducting a geometric morphometric analysis of skull morphology and allometry in target taxa, living and extinct, and elaborate on the relation between skull size and body size. Our analysis indicates that skull size and body size within terrestrial placental mammals scale almost isometrically (PGLS major axis slope 0.906). Furthermore, skull shape in dwarf species differed from both their ancestors and the juveniles of extant species. In insular dwarf hippos, the skull was subject to considerable anatomical reorganisation in response to distinct selection pressures affecting early ontogeny (the “island syndrome”). By contrast, skull shape in adult insular dwarf elephants can be explained well by allometric effects; selection on size may thus have been the main driver of skull shape in dwarf elephants. We suggest that a tightly constrained growth trajectory, without major anatomical reorganization of the skull, allowed for flexible adaptations to changing environments and was one of the factors underlying the evolutionary success of insular dwarf elephants.





Similar content being viewed by others
References
Accordi, F. S., & Palombo, M. R. (1971). Morfologia endocranica degli elephanti nani pleistocenici de Spinagalo (Siracusa) e comparazione con l´endocranio de Elephas antiquus. Rediconti dell´Accademia Nazionale del Lincei (Series 8), 51, 111–124.
Adam, K. D. (1986). Fossilfunde aus den Cannstatter Sauerwasserkalken. In K. D. Adam, W. Reif & E. Wagner (Eds.), Seugnisse des Urmenschen aus den Cannstatter Sauerwasserkalken (Vol. 11, pp. 25–61). Baden-Württemberg: Fundber.
Aguirre, E. (1969). Revision sistematica de los Elephantidae, por su morfologia y morfometria dentaria. Estudios Geologicos, 25, 317–367.
Ambrosetti, P. (1968). The Pleistocene dwarf elephant of Spinagallo. Geologica Romana, 7, 277–398.
Argue, D., Donlon, D., Groves, C., & Wright, R. (2006). Homo floresiensis: Microcephalic, pygmoid, Australopithecus or Homo? Journal of Human Evolution, 51, 360–374.
Argue, D., Groves, C. P., Lee, M. S. Y., & Jungers, W. L. (2017). The afffinities of Homo floresiensis based on phylogenetic analyses of cranial, dental, and postcranial characters. Journal of Human Evolution, 197, 107–133.
Argue, D., Morwood, M., Sutikna, T., Jatmiko, W., & Saptomo, E. (2009). Homo floresiensis: A cladistic analysis. Journal of Human Evolution, 57, 623–639.
Athanassiou, A., Herridge, V., Reese, D. S., et al. (2015). Cranial evidence for the presence of a second endemic elephant species on Cyprus. Quaternary International, 379, 47–57.
Baab, K. L., & McNulty, K. P. (2009). Size, shape, and asymmetry in fossil hominins: The status of the LB1 cranium based on 3D morphometric analyses. Journal of Human Evolution, 57, 608–622.
Bininda-Emonds, O. R. P., Cardillo, M., Jones, K. E., MacPhee, R. D. E., Beck, R. M. D., Grenyer, R., Price, S. A., Vos, R. A., Gittleman, J. L., & Purvis, A. (2007). The delayed rise of present-day mammals. Nature, 446, 507–512.
Boekschoten, G. J., & Sondaar, P. Y. (1972). On the fossil mammalia of Cyprus, I and II. Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen, 75, 306–338.
Boisserie, J. R. (2005). The phylogeny and taxonomy of Hippopotamidae (Mammalia: Artiodactyla): A review based on morphology and cladistic analysis. Zoological Journal of the Linnean Society, 143, 1–26.
Cardini, A., & Polly, P. D. (2013). Larger mammals have longer faces because of size-related constraints on skull form. Nature Communications, 4, 2458. https://doi.org/10.1038/ncomms3458.
Chiozzi, G., Bardelli, G., Ricci, M., De Marchi, G., & Cardini, A. (2014). Just another island dwarf? Phenotypic distinctiveness in the poorly known Soemmerring’s Gazelle, Nanger soemmerringii (Cetartiodactyla: Bovidae), of Dahlak Kebir Island. Biological Journal of the Linnean Society, 111, 603–620.
Coryndon, S. C. (1977). The taxonomy and nomenclature of the Hippopotamidae (Mammalia, Artiodactyla) and a description of two new fossil species. The nomenclature of the Hippopotamidae. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen B, 80, 61–88.
Dryden, I. L., & Mardia, K. M. (2008). Statistical shape analysis. Chicester: Wiley.
Evans, A. R., Jones, D., Boyer, A. G., et al. (2012). The maximum rate of mammal evolution. Proceedings of the National Academy of Sciences of the United States of America, 109, 4187–4190.
Falk, D., Hildebolt, C., Smith, K., et al. (2007). Brain shape in human microcephalics and Homo floresiensis. Proceedings of the National Academy of Sciences of the USA, 104, 2513–2518.
Ferretti, M. P. (2008). The dwarf elephant Palaeoloxodon mnaidriensis from Puntali Cave, Carini (Sicily; late Middle Pleistocene): Anatomy, systematics and phylogenetic relationships. Quaternary International, 182, 90–108.
Fovet, W., Faure, M., & Guerin, C. (2011). Hippopotamus guldbergi n. sp.: révision du statut d'Hippopotamus madagascariensis Guldberg, 1883, après plus d'un siècle de malentendus et deconfusions taxonomiques. Zoosystema, 33, 61–82.
Goodman, S. M., & Jungers, W. L. (2014). Extinct Madagascar: Picturing the Island’s Past. Chicago: University of Chicago Press.
Herridge, V. L. (2010). Dwarf elephants on Mediterranean islands: A natural experiment in parallel evolution. PhD Thesis, University College, London.
Houtekamer, J. L., & Sondaar, P. Y. (1979). Osteology of the fore limb of the Pleistocene dwarf hippopotamus from Cyprus with special reference to phylogeny and function. Proceedings van de Koninklijke Nederlandse Akademie van Wetenschappen, 82, 411–448.
Iinuma, Y. M., Tanaka, S., Kawasaki, K., Kuwajima, T., Nomura, H., Suzuki, M., & Ohtaishi, N. (2004). Dental incremental lines in Sika Deer (Cervus nippon); polarized light and fluorescence microscopy of ground sections. Journal of Veterinary Medical Science, 66, 665–669.
Kaifu, Y., Baba, H., Sutika, T., Morwood, M. J., Kubo, D., Saptomo, W., Jatmiko, E., Due Awe, R., & Djubiantono, T. (2011). Craniofacial morphology of Homo floresiensis: Description, taxonomic affinities, and evolutionary implication. Journal of Human Evolution, 61, 644–682.
Katsikosta, N., & Theodorou, G. (1994). Conservation and reconstruction of a juvenile skull of Palaeoloxodon antiquus falconeri from Charkadio Cave, Tilos island (Dodecanese, Greece). Bulletin of the Speleological Society of Greece, 21, 263–628.
Klingenberg, C. P. (2011). MorphoJ: An integrated software package for geometric morphometrics. Molecular Ecology Resources, 11, 353–357.
Kolb, C., Scheyer, T. M., Veitschegger, K., et al. (2015). Mammalian bone palaeohistology: A survey and new data with emphasis on island forms. PeerJ, 3, e1358. https://doi.org/10.7717/peerj.1358.
Kubo, M. O., Fujita, M., Matsu’ura, S., Kondo, M., & Suwa, G. (2011). Mortality profiles of late Pleistocene deer remains of Okinawa Island: Evidence from the Hananda-Gama cave and Yamashita-cho cave I sites. Anthropological Series, 119, 183–201.
Kurt, F., & Kumarasinghe, J. C. (1998). Remarks on body growth and phenotypes in Asian elephant Elephas maximus. Acta Theriologica, 43, 135–153.
Laws, R. M. (1966). Age criteria for the African elephant Loxodonta africana. East African Wildlife Journal, 4, 1–37.
Laws, R. M. (1968). Dentition and ageing of the Hippopotamus. East African Wildlife Journal, 6, 19–52.
Lister, A. M. (1989). Rapid dwarfing of red deer on Jersey in the Last Interglacial. Nature, 342, 539–542.
Lister, A. M. (1996). Dwarfing in island elephants and deer: Processes in relation to time of isolation. Symposia of the Zoological Society of London, 69, 277–292.
Lomolino, M. V., Sax, D. F., Palombo, M. R., & van der Geer, A. A. E. (2012). Of Mice and mammoths: Evaluations of causal explanations for body size evolution in insular mammals. Journal of Biogeography, 39, 842–854.
Lomolino, M. V., van der Geer, A. A. E., Lyras, G. A., Palombo, M. R., Sax, D. F., & Rozzi, R. (2013). Of mice and mammoths: Generality and antiquity of the island rule. Journal of Biogeography, 40, 1427–1439.
Lyras, G. A., Dermitzakis, M. D., van der Geer, A. A. E., & de Vos, J. (2009). The origin of Homo floresiensis and its relation to evolutionary processes under isolation. Anthropological Science, 117, 33–43.
Lyras, G. A., van der Geer, A. A. E., & Rook, L. (2010). Body size of insular carnivores: Evidence from the fossil record. Journal of Biogeography, 37, 1007–1021.
Lyras, G. A., van der Geer, A. E., Dermitzakis, M., & de Vos, J. (2006). Cynotherium sardous, an insular canid (Mammalia: Carnivora) from the Pleistocene of Sardinia (Italy), and its origin. Journal of Vertebrate Paleontology, 26, 735–745.
Martin, R. B. (2005). The Transboundary Mammal Project of the Ministry of Environment and Tourism, Namibia (p. 74). Windhoek: The Namibia Nature Foundation.
Martinez, A., & Hamsici, O. (2008). Who is LB1? Discriminant analysis for the classification of specimens. Pattern Recognition, 41, 3436–3441.
Masters, J. C., Génin, F., Silvestro, D., Lister, A. M., & DelPero, M. (2014). The red island and the seven dwarfs: Body size reduction in Cheirogaleidae. Journal of Biogeography, 41, 1833–1847.
Millien, V. (2006). Morphological evolution is accelerated among island mammals. PLoS Biology, 4, e321.
Mitteroecker, P., Gunz, P., & Bookstein, F. L. (2005). Heterochrony and geometric morphometrics: A comparison of cranial growth in Pan paniscus versus Pan troglodytes. Evolution & Development, 7, 244–258.
Mitteroecker, P., Gunz, P., Windhager, S., & Schaefer, K. (2013). A brief review of shape, form, and allometry in geometric morphometrics, with applications to human facial morphology. Hystrix, The Italian Journal of Mammalogy, 24, 59–66.
O’Higgins, P., & Jones, N. (1998). Morphologika a program for the analysis of 3-dimensional shape variation using landmarks. http://discovery.ucl.ac.uk/id/eprint/172805.
Palkopoulou, E., Lipson, M., Mallick, S., Nielsen, S., Rohland, N., Baleka, S., et al. (2018). A comprehensive genomic history of extinct and living elephants. Proceedings of the National Academy of Sciences of the USA. https://doi.org/10.1073/pnas.1720554115.
Palombo, M. R. (2001). Paedomorphic features and allometric growth in the skull of Elephas falconeri from Spinagallo (Middle Pleistocene, Sicily). In G. Cavaretta, P. Gioia, M. Mussi & M. R. Palombo (Eds), The World of Elephants. Proceedings of the First International Congress, Rome, (Vol. 16–20, pp. 492–496). Roma: CNR.
Penin, X., Berge, C., & Baylac, M. (2002). Ontogenetic study of the skull in modern humans and the common chimpanzees: Neotenic hypothesis reconsidered with a tridimensional procrustes analysis. American Journal of Physical Anthropology, 118, 50–62.
Pergams, O. R. W., & Ashley, M. V. (1999). Rapid morphological change in island deer mice. Evolution, 53, 1573–1581.
Revell, L. J. (2009). Size-correction and principal components for interspecific comparative studies. Evolution, 63, 3258–3268.
Roca, A. L., Georgiadis, N., Pecon-Slattery, J., & O’Brien, S. J. (2001). Genetic evidence for two species of elephant in Africa. Science, 293, 1473–1477.
Roth, V. L. (1984). How elephants grow: Heterochrony and the calibration of developmental stages in some living and fossil species. Journal of Vertebrate Paleontology, 4, 126–145.
Roth, V. L. (1992). Inferences from allometry and fossils: Dwarfing of elephants on islands. Oxford Surveys in Evolutionary Biology, 8, 259–288.
Roth, V. L. (1993). Dwarfism and variability in the Santa Rosa Island mammoth (Mammuthus exilis): An interspecific comparison of limb-bone sizes and shapes in elephants. In F. G. Hochberg (Ed.), Third California Islands Symposium (pp. 433–442). Santa Barbara: Santa Barbara Museum of Natural History.
Roth, V. L., & Shoshani, J. (1988). Dental identification and age determination in Elephas maximus. Journal of Zoology, 214, 288–567.
Sikes, K. S. (1967). The African elephant, Loxodonta africana: A field method for the estimation of age. Journal of Zoology, 154, 235–248.
Smaers, J. B. (2014). Evomap: R-package for the evolutionary mapping of continuous traits. Available at Github: https://github.com/JeroenSmaers/evomap.
Smith, F. A., Lyons, S. K., Ernest, S. K. M., Jones, K. E., Kaufman, D. M., Dayan, T., Marquet, P. A., Brown, J. H., & HaskelI, J. P. (2003). Body mass of late Quaternary mammals. Ecology, 84, 3402.
Sondaar, P. Y. (1977). Insularity and its effect on mammal evolution. In M. K. Hecht, P. C. Goody & B. M. Hecht (Eds), Major Patterns in Vertebrate Evolution (pp. 671–707). New York: Plenum Publications Corporation.
Sondaar, P. Y. (1994). Paleoecology and evolutionary patterns in horses and island mammals. Historical Biology, 8, 1–13.
Stuenes, S. (1989). Taxonomy, habits, and relationships of the subfossil Madagascan Hippopotami Hippopotamus lemerlei and H. madagascariensis.. Journal of Vertebrate Paleontology, 9, 241–268.
Todd, N. (2010). Qualitative Comparison of the Cranio-Dental Osteology of the Extant Elephants, Elephas Maximus (Asian Elephant) and Loxodonta africana (African Elephant). The Anatomical Record, 293, 62–73.
van den Bergh, G. D., Kaifu, Y., Kurniawan, I., et al. (2016). Homo floresiensis-like fossils from the early Middle Pleistocene of Flores. Nature, 534, 245–248.
van den Bergh, G. D., Meijer, H. J. M., Awe, R. D. et al. (2009). The Liang Bua faunal remains: A 95k.yr. sequence from Flores, East Indonesia. Journal of Human Evolution, 57, 527–537.
van der Geer, A., Lyras, G., de Vos, J., & Dermitzakis, M. (2010). Evolution of Island Mammals: Adaptation and Extinction of Placental Mammals on Islands (p. 479). Oxford: Wiley-Blackwell Publishing.
van der Geer, A. A. E. (2005). Island ruminants and the evolution of parallel functional structures. In E. Crégut (Ed.) Les ongules holarctiques du Pliocene et du Pleistocene. Actes Colloque International Avignon, (Vol. 19–22, pp. 231–240) Quaternair (hors-serie 2).
van der Geer, A. A. E. (2014). Parallel patterns and trends in functional structures in island mammals. Integrative Zoology, 9, 167–182.
van der Geer, A. A. E., Lyras, G. A., Lomolino, M. V., Palombo, & Sax, M. R., D.F (2013). Body size evolution of palaeo-insular mammals: Temporal variations and interspecific interactions. Journal of Biogeography, 40, 1440–1450.
van der Geer, A. A. E., Lyras, G. A., van den Hoek Ostende, L. W., de Vos, J., & Drinia, H. (2014). A dwarf elephant and a rock mouse on Naxos (Cyclades, Greece) with a revision of the palaeozoogeography of the Cycladic Islands (Greece) during the Pleistocene. Palaeogeography, Palaeoclimatology, Palaeoecology, 404, 133–144.
van der Geer, A. A. E., van den Bergh, G. D., Lyras, G. A., Prasetyo, U. W., Awe Due, R., Setiyabudi, E., & Drinia, H. (2016). The effect of area and isolation on insular dwarf proboscideans. Journal of Biogeography, 43, 1656–1666.
van Heteren, A. H. (2008). Homo floresiensis is an island form. PalArch’s Journal of Vertebrate Palaeonotology, 5(2), 1–12.
Van Valen, L. (1973). Body size and numbers of plants and animals. Evolution, 27, 27–35.
Weston, E. M. (1998). A biometrical analysis of evolutionary change within the Hippopotamidae. PhD thesis, University of Cambridge, England.
Weston, E. M. (2003). Evolution of ontogeny in the hippopotamus skull: Using allometry to dissect developmental change. Biological Journal of the Linnean Society, 80, 625–638.
Weston, E. M., & Lister, A. M. (2009). Insular dwarfism in hippos and a model for brain size reduction Homo floresiensis. Nature, 459, 85–88.
Zeitoun, V., Barriel, V., & Widianto, H. (2016). Phylogenetic analysis of the calvaria of Homo floresiensis. Comptes Rendus Palevol, 15, 555–568.
Acknowledgements
We thank Steven van der Mije and Wendy van Bohemen (RMNH), Eileen Westwig and Neil Duncan (AMNH), Laurence Heaney and the late William Stanley (FMNH), Carolina di Patti (MGG), Oliver Hampe, Frieder Mayer and Nora Lange (MFN), Christine Argot and Christine Lefèvre (MNHN), Rainer Brocke and Christine Hertler (SFN), Reinhard Ziegler (SMNS), Darrin Lunde and Nicole Edmison (USNM), Chloe Adamopoulou (ZMUA), Pasquale Raia and Mariella Del Re (MPUN) and Pip Brewer (Natural History Museum, London) for allowing us to study the skulls in their care and their assistance when skulls were too heavy to handle two-handed. We further thank Rutger Vos for clarifying PGLS regressions, and Gert van den Bergh (Centre for Archaeological Science, University of Wollongong), Maria Rita Palombo (‘La Sapienza’ University of Rome), Athanassios Athanassiou (Hellenic Ministery of Culture), Adrian Lister, Chris Stringer and Victoria Herridge (Natural History Museum, London) for discussions we had on island dwarfs.
Funding
GL received support from the SYNTHESYS Project (GB-TAF-6355 and FR-TAF-6549). The research of AVDG has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: THALIS—UOA-Island biodiversity and cultural evolution: examples from the Eastern Mediterranean, Madagascar, Mauritius and Philippines during the past 800,000 years (MIS375910, KA:70/3/11669).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van der Geer, A.A.E., Lyras, G.A., Mitteroecker, P. et al. From Jumbo to Dumbo: Cranial Shape Changes in Elephants and Hippos During Phyletic Dwarfing. Evol Biol 45, 303–317 (2018). https://doi.org/10.1007/s11692-018-9451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-018-9451-1