Abstract
Introduction
Owing to evolution of parasite strains that are resistant to existing antimalarial drugs, research for novel antimalarial medicines is progressing on numerous fronts.
Purpose
Herein, we evaluated the in vivo anti-Plasmodium berghei activity of β-ionone including its ameliorative potential towards P. berghei-associated anaemia and oxidative organ damage.
Methods
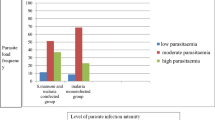
Mice were infected with chloroquine-sensitive strain of P. berghei and then treated with β-ionone at doses of 10 and 20 mg/kg body weight (BW) for seven days. The parasitemia, packed cell volume and redox sensitive biomarkers in the liver, brain and spleen were estimated.
Results
Our result showed that β-ionone, in a dose-dependent fashion, significantly (p < 0.05) repressed the multiplication of P. berghei. More so, the compound, at doses of 10 and 20 mg/kg BW, significantly (p < 0.05) mitigated anaemia and organ damage induced by P. berghei.
Conclusion
Overall, the findings demonstrated that β-ionone has antiplasmodial actions and plays a mitigative role against P. berghei-induced anaemia and oxidative organ damage.



Similar content being viewed by others
Data Availability
Not applicable.
References
White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM (2014) Malaria. Lancet 383:723–735
World Health Organization (2022) World malaria report: 2022, WHO: Geneva, Switzerland. p 293. https://www.who.int/publications/i/item/9789240064898. Accessed 1 Oct 2023
World Health Organization (2010) World malaria report: 2010, WHO: Geneva, Switzerland. p 205. https://apps.who.int/iris/handle/10665/44451. Accessed 9 May 2022
Postma NS, Zuidema J, Mommérs EC, Eling WM (1996) Oxidative stress in malaria; implications for prevention and therapy. Pharm World Sci 18(4):121–129. https://doi.org/10.1007/BF00717727
Isah MB, Ibrahim MA (2014) The role of antioxidants treatment on the pathogenesis of malarial infections: a review. Parasitol Res 113(3):801–809. https://doi.org/10.1007/s00436-014-3804-1
White NJ (2004) Antimalarial drug resistance. J Clin Invest 113(8):1084–1092. https://doi.org/10.1172/JCI21
Ouji M, Augereau JM, Paloque L, Benoit-Vical F (2018) Plasmodium falciparum resistance to artemisinin-based combination therapies: a sword of Damocles in the path toward malaria elimination. Parasite 25:24. https://doi.org/10.1051/parasite/2018021
Gomes-Carneiro MR, De-Oliveira AC, De-Carvalho RR, Araujo IB, Souza CA, Kuriyama SN, Paumgartten FJ (2003) Inhibition of cyclophosphamide-induced teratogenesis by β-ionone. Toxicol Lett 138(3):205–213. https://doi.org/10.1016/S0378-4274(02)00413-7
Nacke C, Hüttmann S, Etschmann MM, Schrader J (2012) Enzymatic production and in situ separation of natural β-ionone from β-carotene. J Ind Microbiol Biotechnol 39(12):1771–1778. https://doi.org/10.1007/s10295-012-1182-1
Gomes-Carneiro MR, Dias DM, Paumgartten FJ (2006) Study on the mutagenicity and antimutagenicity of β-ionone in the Salmonella/microsome assay. Food Chem Toxicol 44(4):522–527. https://doi.org/10.1016/j.fct.2005.08.026
Markovich YD, Panfilov AV, Platunov YN, Zhirov AA, Kosenko SI, Kirsanov AT (1998) New cyclization agents for the synthesis of beta-ionone from pseudoionone. Pharm Chem J 32(11):603–605. https://doi.org/10.1007/BF02465835
Sharma V, Singh G, Kaur H, Saxena AK, Ishar MP (2012) Synthesis of β-ionone derived chalcones as potent antimicrobial agents. Bioorg Med Chem Lett 22(20):6343–6346. https://doi.org/10.1016/j.bmcl.2012.08.084
Balbi A, Anzaldi M, Mazzei M, Miele M, Bertolotto M, Ottonello L, Dallegri F (2006) Synthesis and biological evaluation of novel heterocyclic ionone-like derivatives as anti-inflammatory agents. Bioorg Med Chem 14(15):5152–5160. https://doi.org/10.1016/j.bmc.2006.04.007
Duncan RE, Lau D, El-Sohemy A, Archer MC (2004) Geraniol and β-ionone inhibit proliferation, cell cycle progression, and cyclin-dependent kinase 2 activity in MCF-7 breast cancer cells independent of effects on HMG-CoA reductase activity. Biochem Pharmacol 68(9):1739–1747. https://doi.org/10.1016/j.bcp.2004.06.022
Liu JR, Chen BQ, Yang BF, Dong HW, Sun CH, Wang Q et al (2004) Apoptosis of human gastric adenocarcinoma cells induced by β-ionone. World J Gastroenterol 10(3):348. https://doi.org/10.3748/wjg.v10.i3.348
Janakiram NB, Cooma I, Mohammed A, Steele VE, Rao CV (2008) β-Ionone inhibits colonic aberrant crypt foci formation in rats, suppresses cell growth, and induces retinoid X receptor-α in human colon cancer cells. Mol Cancer Ther 7(1):181–190. https://doi.org/10.1158/1535-7163.MCT-07-0529
Faezizadeh Z, Gharib A, Goudarzi M (2015) The effect of β-ionone on telomerase activity in the human leukemia cell line K562. J Kerman Univ Med Sci 19(3):e69871. https://doi.org/10.22110/jkums.v19i3.2160
Suryawanshi SN, Bhat BA, Pandey S, Chandra N, Gupta S (2007) Chemotherapy of leishmaniasis. Part VII: synthesis and bioevaluation of substituted terpenyl pyrimidines. Eur J Med Chem 42(9):1211–1217. https://doi.org/10.1016/j.ejmech.2006.10.002
Aminu S, Ibrahim MA, Dada Chechet G, Onyike E (2022) Chemotherapeutic potentials of β-ionone against Trypanosoma congolense infection: Inhibition of parasite proliferation, anemia development, trans-sialidase (TconTS3 and TconTS4) gene expressions, and phospholipase A2. Chem Biol Drug Des 99(6):908–922. https://doi.org/10.1111/cbdd.14048
Usman MA, Usman FI, Abubakar MS, Salman AA, Adamu A, Ibrahim MA (2021) Phytol suppresses parasitemia and ameliorates anaemia and oxidative brain damage in mice infected with Plasmodium berghei. Exp Parasitol 224:108097. https://doi.org/10.1016/j.exppara.2021.108097
Niehaus WG Jr, Samuelsson B (1968) Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6(1):126–130. https://doi.org/10.1111/j.1432-1033.1968.tb00428.x
Akanji MA, Adeyemi OS, Oguntoye SO, Sulyman F (2009) Psidium guajava extract reduces trypanosomosis associated lipid peroxidation and raises glutathione concentrations in infected animals. EXCLI J 8(1):148–154
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82(1):70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Fridovich I (1975) Superoxide dismutases. Annu Rev Biochem 44(1):147–159
Bushby SR, Hitchings GH (1968) Trimethoprim, a sulphonamide potentiator. Br J Pharmacol Chemother 33(1):72–90. https://doi.org/10.1111/j.1476-5381.1968.tb00475.x
Darrell JH, Garrod LP, Waterworth PM (1968) Trimethoprim: laboratory and clinical studies. J Clin Pathol 21(2):202. https://doi.org/10.1136/jcp.21.2.202
Fasan PO (1971) Trimethoprim plus sulphamethoxazole compared with chloroquine in the treatment and suppression of malaria in African schoolchildren. Ann Trop Med Parasitol 65(1):117–121. https://doi.org/10.1080/00034983.1971.11686737
Haldar K, Mohandas N (2009) Malaria, erythrocytic infection, and anemia. Am Soc Hematolo Educ Program Book 1:87–93. https://doi.org/10.1182/asheducation-2009.1.87
Rashid MK, Alam R, Khan S, Prakash V (2013) Oxidative stress marker and antioxidant status in falciparum malaria in relation to the intensity of parasitemia. Int J Biol Med Res 4(3):3469–3471
Liu JR, Dong HW, Sun XR, Wang Q, Sun WG, Parry JW et al (2009) Effects of β-ionone on mammary carcinogenesis and antioxidant status in rats treated with DMBA. Nutr Cancer 62(1):58–65. https://doi.org/10.1080/01635580903191510
Asokkumar S, Naveenkumar C, Raghunandhakumar S, Kamaraj S, Anandakumar P, Jagan S et al (2012) Antiproliferative and antioxidant potential of beta-ionone against benzo (a) pyrene-induced lung carcinogenesis in Swiss albino mice. Mol Cell Biochem 363(1):335–345. https://doi.org/10.1007/s11010-011-1186-6
Acknowledgements
The authors are grateful to Dr. Mohammed Auwal Ibrahim, Mr. Suleiman Aminu and the management of Ahmadu Bello University, Zaria, Nigeria for critical review of the manuscript, provision of β-ionone and study facilities, respectively.
Funding
None received.
Author information
Authors and Affiliations
Contributions
MAU and MAS: conceptualised the research study and designed the experiments. FBI, HM, SOA and UAI: conducted all the laboratory experiments. MAU and MAS: supervised the work. MAU, FBI, H-OM, SOA and UAI: analyzed the data. MAU: wrote the manuscript. MAS, FBI, H-OM, SOA and UAI: revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest reported by the authors in relation to this study.
Ethical Approval
All procedures conducted in this study were based on the ethical standards of the institutional research committee on animal experimentation.
Informed Consent
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Usman, M.A., Ibrahim, F.B., Mohammed, HO. et al. Antiplasmodial Activity of β-Ionone and the Effect of the Compound on Amelioration of Anaemia and Oxidative Organ Damage in Mice Infected with Plasmodium berghei. Acta Parasit. 69, 242–250 (2024). https://doi.org/10.1007/s11686-023-00741-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00741-7