Abstract
Purpose
Neosporosis is an important widespread parasitic infection caused by N. caninum. It infects a wide range of warm-blooded animals as intermediate hosts and dogs as the definitive host. Nevertheless, there are a number of questions regarding the life cycle and epidemiological aspects of N. caninum. Also, the role of felids (domestic and non-domestic) in the life cycle of N. caninum has been little described. Therefore, this study was conducted to evaluate the global prevalence of N. caninum in domestic and wild felids.
Methods
PubMed, Scopus, Google Scholar, Web of Science, and ScienceDirect databases were searched for articles published on the prevalence of N. caninum in felids until Aprill 2, 2022 and the reference lists of retrieved articles were screened. A random-effects meta-analysis model was used to estimate the pooled prevalence and 95% confidence interval. Heterogeneity among studies was evaluated using Cochran’s Q and the I2 statistic.
Results
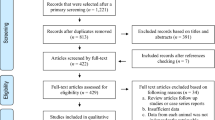
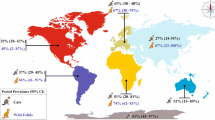
After exclusion of irrelevant articles and duplication removal, 30 studies were eligible for quantitative analysis including 20 studies on domestic cats and 10 studies on wild felids. The overall prevalence of neosporosis infection in cats was 15% (95% CI 10–21%) that was significantly higher in wild felids (26%, 95% CI 13–38%) than in domestic cats (11%, 95% CI 6–16%) (P = 0.03). There was no significant difference in pooled prevalence between male and female domestic cats (P = 0.75). Regarding continent, the lowest prevalence of neosporosis infection was in Asia (9%, 95% CI 1–20%) and the highest was in North America (43.6%, 95% CI 33.9–53.2%) and Africa (18%, 95% CI 9–46%). Higher prevalence was obtained when using the NAT with 22% (95% CI 7–37%), compared to the IFAT (17%, 95% CI 9–24%) and ELISA (6%, 95% CI 2–9%) (P = 0.01).
Conclusion
The findings highlighted the importance of felids as potential intermediate hosts of neosporosis despite the fact that the source of the parasite for these animals was unknown. Further studies should be performed to investigate the role of this top predator (felids) in maintaining both domestic and sylvatic cycles of Neospora caninum.





Similar content being viewed by others
Availability of Data and Material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Dubey J, Schares G, Ortega-Mora L (2007) Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev 20:323–367. https://doi.org/10.1128/CMR.00031-06
Donahoe SL, Lindsay SA, Krockenberger M et al (2015) A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int J Parasitol Parasites Wildl 4:216–238. https://doi.org/10.1016/j.ijppaw.2015.04.002
Marugan-Hernandez V (2017) Neospora caninum and bovine neosporosis: current vaccine research. J Comp Pathol 157:193–200. https://doi.org/10.1016/j.jcpa.2017.08.001
Sazmand A, Bahari A, Papi S, Otranto D (2020) Parasitic diseases of equids in Iran (1931–2020): a literature review. Parasit Vectors 13:1–19. https://doi.org/10.1186/s13071-020-04472-w
Marsh A, Howe D, Wang G et al (1999) Differentiation of Neospora hughesi from Neospora caninum based on their immunodominant surface antigen, SAG1 and SRS2. Int J Parasitol 29:1575–1582. https://doi.org/10.1016/s0020-7519(99)00120-4
Silva RC, Machado GP (2016) Canine neosporosis: perspectives on pathogenesis and management. Vet Med (Auckl) 7:59. https://doi.org/10.2147/VMRR.S76969
Rodrigues AA, Reis SS, de Sousa ML et al (2020) A systematic literature review and meta-analysis of risk factors for Neospora caninum seroprevalence in goats. Prev Vet Med 185:105176. https://doi.org/10.1016/j.prevetmed.2020.105176
Ansari-Lari M (2021) Neospora caninum in aborted bovine fetuses in Iran: a systematic review and meta-analysis. Ann Parasitol 67:357–366. https://doi.org/10.17420/ap6703.351
Morales LFM, Lagorio V, Corigliano MG et al (2022) Neosporosis in sheep: a systematic review and meta-analysis of global seroprevalence and related risk factors. Acta Trop. https://doi.org/10.1016/j.actatropica.2022.106569
Reichel MP, McAllister MM, Nasir A, Moore DP (2015) A review of Neospora caninum in water buffalo (Bubalus bubalis). Vet parasitol 212:75–79. https://doi.org/10.1016/j.vetpar.2015.08.008
Anvari D, Saberi R, Sharif M et al (2020) Seroprevalence of Neospora caninum infection in dog population worldwide: a systematic review and meta-analysis. Acta Parasitol 65:273–290. https://doi.org/10.2478/s11686-019-00163-4
Lamberski N (2015) Felidae. J Zoo Wildl Med. https://doi.org/10.1016/B978-1-4557-7397-8.00047-5
Trouwborst A, McCormack PC, Martínez Camacho E (2020) Domestic cats and their impacts on biodiversity: a blind spot in the application of nature conservation law. People Nature 2:235–250. https://doi.org/10.1002/pan3.10073
Dubey J, Lindsay D, Lipscomb T (1990) Neosporosis in cats. Vet Pathol 27:335–339. https://doi.org/10.1177/030098589002700505
Duarte PO, Oshiro LM, Zimmermann NP et al (2020) Serological and molecular detection of Neospora caninum and Toxoplasma gondii in human umbilical cord blood and placental tissue samples. Sci Rep 10:1–8. https://doi.org/10.1038/s41598-020-65991-1
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269. https://doi.org/10.7326/0003-4819-151-4-200908180-00135
Cheadle MA, Spencer JA, Blagburn BL (1999) Seroprevalences of Neospora caninum and Toxoplasma gondii in nondomestic felids from southern Africa. J Zoo Wildl Med 30:248–251
Ribeiro TMP, Reis TS, Sousa SAP et al (2022) Antibody frequency for Toxoplasma gondii and Neospora spp. in domiciliated and stray cats from Araguaína, Tocantins. Eastern Amazonia Semin Cienc Agrar 43:629–640. https://doi.org/10.5433/1679-0359.2022v43n2p629
André M, Adania C, Teixeira R et al (2010) Antibodies to Toxoplasma gondii and Neospora caninum in captive neotropical and exotic wild canids and felids. J Parasitol 96:1007–1009. https://doi.org/10.1645/GE-2502.1
Bresciani KDS, Gennari S, Serrano A et al (2007) Antibodies to Neospora caninum and Toxoplasma gondii in domestic cats from Brazil. Parasitol Res 100:281–285. https://doi.org/10.1007/s00436-006-0262-4
Ferroglio E, Guiso P, Pasino M et al (2005) Antibodies to Neospora caninum in stray cats from north Italy. Vet parasitol 131:31–34. https://doi.org/10.1016/j.vetpar.2005.04.012
Hamidinejat H, Mosalanejad B, Avizeh R et al (2011) Neospora caninum and Toxoplasma gondii antibody prevalence in Ahvaz feral cats. Iran Jundishapur J Microbiol 4:217–222
Silaghi C, Knaus M, Rapti D et al (2014) Survey of Toxoplasma gondii and Neospora caninum, haemotropic mycoplasmas and other arthropod-borne pathogens in cats from Albania. Parasit Vectors 7:1–10. https://doi.org/10.1186/1756-3305-7-62
Arraes-Santos AI, Araújo AC, Guimarães MF et al (2016) Seroprevalence of anti-Toxoplasma gondii and anti-Neospora caninum antibodies in domestic mammals from two distinct regions in the semi-arid region of Northeastern Brazil. Vet Parasitol Reg Stud Reports 5:14–18. https://doi.org/10.1016/j.vprsr.2016.08.007
Braga MdSCdO, André MR, Jusi MMG et al (2012) Occurrence of anti-Toxoplasma gondii and anti-Neospora caninum antibodies in cats with outdoor access in São Luís, Maranhão, Brazil. Rev Bras Parasitol Vet 21:107–111. https://doi.org/10.1590/s1984-29612012000200007
Coelho WMD, do Amarante AFT, Apolinário JdC, et al (2011) Seroepidemiology of Toxoplasma gondii, Neospora caninum, and Leishmania spp. infections and risk factors for cats from Brazil. Parasitol Res 109:1009–1013. https://doi.org/10.1007/s00436-011-2461-x
Feitosa TF, Vilela VLR, Dantas ES et al (2014) Toxoplasma gondii and Neospora caninum in domestic cats from the Brazilian semi-arid: seroprevalence and risk factors. Arq Bras Med Vet Zootec 66:1060–1066. https://doi.org/10.1590/1678-6696
Hornok S, Edelhofer R, Joachim A et al (2008) Seroprevalence of Toxoplasma gondii and Neospora caninum infection of cats in Hungary. Acta Vet Hung 56:81–88. https://doi.org/10.1556/AVet.56.2008.1.8
Meneses IDSd, Andrade MR, Uzêda RS et al (2014) Frequency of antibodies against Sarcocystis neurona and Neospora caninum in domestic cats in the state of Bahia, Brazil. Rev Bras Parasitol Vet 23:526–529. https://doi.org/10.1590/S1984-29612014080
Munhoz AD, Hage SB, Cruz RDS et al (2017) Toxoplasmosis in cats in northeastern Brazil: Frequency, associated factors and coinfection with Neospora caninum, feline immunodeficiency virus and feline leukemia virus. Vet Parasitol Reg Stud Reports 8:35–38. https://doi.org/10.1016/j.vprsr.2017.01.007
Sedlák K, Bartova E, Machacova T (2014) Seroprevalence of Neospora caninum in cats from the Czech Republic. Acta Parasitol 59:359–361. https://doi.org/10.2478/s11686-014-0246-y
Sousa KCMd, Herrera HM, Domingos IH et al (2014) Serological detection of Toxoplasma gondii, Leishmania infantum and Neospora caninum in cats from an area endemic for leishmaniasis in Brazil. Rev Bras Parasitol Vet 23:449–455. https://doi.org/10.1590/S1984-29612014078
de Oliveira KM, Laskoski LM, de Aguiar DM et al (2019) Detection of antibodies against Sarcocystis neurona, Neospora caninum and Toxoplasma gondii in horses, dogs and cat. Braz J Vet Res Anim Sci 56:e152918–e152918. https://doi.org/10.11606/issn.1678-4456.bjvras.2019.152918
Ebani VV, Nardoni S, Maestrini M et al (2021) Serological Survey on the Occurrence of Rickettsia spp., Neospora caninum, Bartonella henselae and Toxoplasma gondii in Cats from Tuscany (Central Italy). Animal 11:1842. https://doi.org/10.3390/ani11061842
Marková J, Machačová T, Bártová E et al (2019) Toxoplasma gondii, Neospora caninum and Encephalitozoon cuniculi in animals from captivity (zoo and circus animals). J Eukaryot Microbiol 66:442–446. https://doi.org/10.1111/jeu.12688
Onuma SSM, Melo ALT, Kantek DLZ et al (2014) Exposure of free-living jaguars to Toxoplasma gondii, Neospora caninum and Sarcocystis neurona in the Brazilian Pantanal. Rev Bras Parasitol Vet 23:547–553. https://doi.org/10.1590/S1984-29612014077
Sedlák K, Bártová E (2006) Seroprevalences of antibodies to Neospora caninum and Toxoplasma gondii in zoo animals. Vet parasitol 136:223–231. https://doi.org/10.1016/j.vetpar.2005.11.021
Spencer JA, Higginbotham MJ, Blagburn BL (2003) Seroprevalence of Neospora caninum and Toxoplasma gondii in captive and free-ranging nondomestic felids in the United States. J Zoo Wildl Med 34:246–249. https://doi.org/10.1638/02-046
Dubey J, Lindsay DS, Hill D et al (2002) Prevalence of antibodies to Neospora caninum and Sarcocystis neurona in sera of domestic cats from Brazil. J Parasitol 88:1251–1252. https://doi.org/10.1645/0022-3395(2002)088[1251:POATNC]2.0.CO;2
Ferroglio E, Wambwa E, Castiello M et al (2003) Antibodies to Neospora caninum in wild animals from Kenya, East Africa. Vet parasitol 118:43–49. https://doi.org/10.1016/j.vetpar.2003.09.006
de Lima DCV, Magalhães FJR, Andrade MR et al (2018) Anti-Neospora caninum antibodies in feral cats on the Island of Fernando de Noronha, Brazil. Acta Parasitol 63:645–646. https://doi.org/10.1515/ap-2018-0074
Arunvipas P, Inpankaew T, Jittapalapong S (2012) Risk factors of Neospora caninum infection in dogs and cats in dairy farms in Western Thailand. Trop Anim Health Prod 44:1117–1121. https://doi.org/10.1007/s11250-011-0048-2
Li S, Lu X, Qiao H et al (2019) First report on Toxoplasma gondii and Neospora caninum Seroprevalence in cats and dogs in Shijiazhuang. China J Glob Epidemiol Environ Health 2020:01–05
Millán J, Cabezón O, Pabón M et al (2009) Seroprevalence of Toxoplasma gondii and Neospora caninum in feral cats (Felis silvestris catus) in Majorca, Balearic Islands, Spain. Vet parasitol 165:323–326. https://doi.org/10.1016/j.vetpar.2009.07.014
Sobrino R, Dubey J, Pabón M et al (2008) Neospora caninum antibodies in wild carnivores from Spain. Vet parasitol 155:190–197. https://doi.org/10.1016/j.vetpar.2008.05.009
Seltmann A, Schares G, Aschenborn OH et al (2020) Species-specific differences in Toxoplasma gondii, Neospora caninum and Besnoitia besnoiti seroprevalence in Namibian wildlife. Parasit Vectors 13:1–12. https://doi.org/10.1186/s13071-019-3871-3
Javanmardi E, Majidiani H, Shariatzadeh SA et al (2020) Global seroprevalence of Neospora spp. in horses and donkeys: a systematic review and meta-analysis. Vet parasitol 288:109299. https://doi.org/10.1016/j.vetpar.2020.109299
Mamaghani AJ, Fathollahi A, Arab-Mazar Z et al (2022) Toxoplasma gondii vaccine candidates: a concise review. Ir J Med Sci. https://doi.org/10.1007/s11845-022-02998-9
Nazari N, Shojaee S, Salimi M et al (2020) Serological survey of Neospora caninum and Toxoplasma gondii co-infection in rodents in Northwestern Iran. Iran J Parasitol 15:253
Nazari N, Bozorgomid A, Janbakhsh A, Bashiri F (2018) Toxoplasma gondii and human immunodeficiency virus co-infection in western Iran: a cross sectional study. Asian Pac J Trop Dis 11:58
Ghalmi F, China B, Jenkins M et al (2014) Comparison of different serological methods to detect antibodies specific to Neospora caninum in bovine and canine sera. J Vet Diagn Invest 26:136–140. https://doi.org/10.1177/1040638713515480
Acknowledgements
We thank the scientists and personnel of the Medical Parasitology Department in Kermanshah University of Medical Sciences, Kermanshah, for their collaboration. We also extend our thanks to clinical research development center of Imam Reza Hospital for their kind advice.
Funding
This study was supported by a grant from the Vice Chancellery for Research and Technology, Kermanshah University of Medical Sciences (grant number: 4010418).
Author information
Authors and Affiliations
Contributions
All authors contributed to study design. AB contributed to all parts of the study. NN and SF contributed to study implementation. MTS, MTK, YH, SAK and SR collaborated in the analysis and interpretation of data. SR, and AB collaborated in the manuscript writing and revision. All the authors commented on the drafts of the manuscript and approved the final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol was approved by the Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.MED.REC.1401.093).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nazari, N., Khodayari, M.T., Hamzavi, Y. et al. Systematic Review and Meta-analysis of Role of Felids as Intermediate Hosts in the Life Cycle of Neospora caninum Based on Serological Data. Acta Parasit. 68, 266–276 (2023). https://doi.org/10.1007/s11686-023-00661-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00661-6